Figure 5. Chaperone activity of
αAG98R-crystallin monomers measured with different substrates. The
assays were performed as described under methods. Nine μg of the
mutant and 10 μg of the wild-type proteins were used in assays. A:
Thermal
aggregation of ADH in the absence or presence of wild-type or
αAG98R-crystallin monomer at 37 °C. In each experiment 75 μg of
ADH was used. (Red, ADH; blue, + αAG98R-crystallin; green, + wt
αA-crystallin). B: Thermal aggregation of βB2-crystallin in the
presence of αAG98R-crystallin monomers. In each experiment 150 μg of
βB2-crystallin was used. (Red, βB2; blue,
+αAG98R-crystallin; green, + wt αA-crystallin). C: Thermal
aggregation of CS in the presence and absence of αAG98R-crystallin
monomers. In each experiment 75 μg of CS was used. (Red, CS; blue,
+αAG98R-crystallin; green,+ wt αA-crystallin). D: Thermal
aggregation of ovotransferrin in presence or absence of
αAG98R-crystallin monomers. In each experiment 100 μg of ovotransferrin
was used. (Red, ovotransferrin; blue, +αAG98R-crystallin; green, + wt
αA-crystallin).
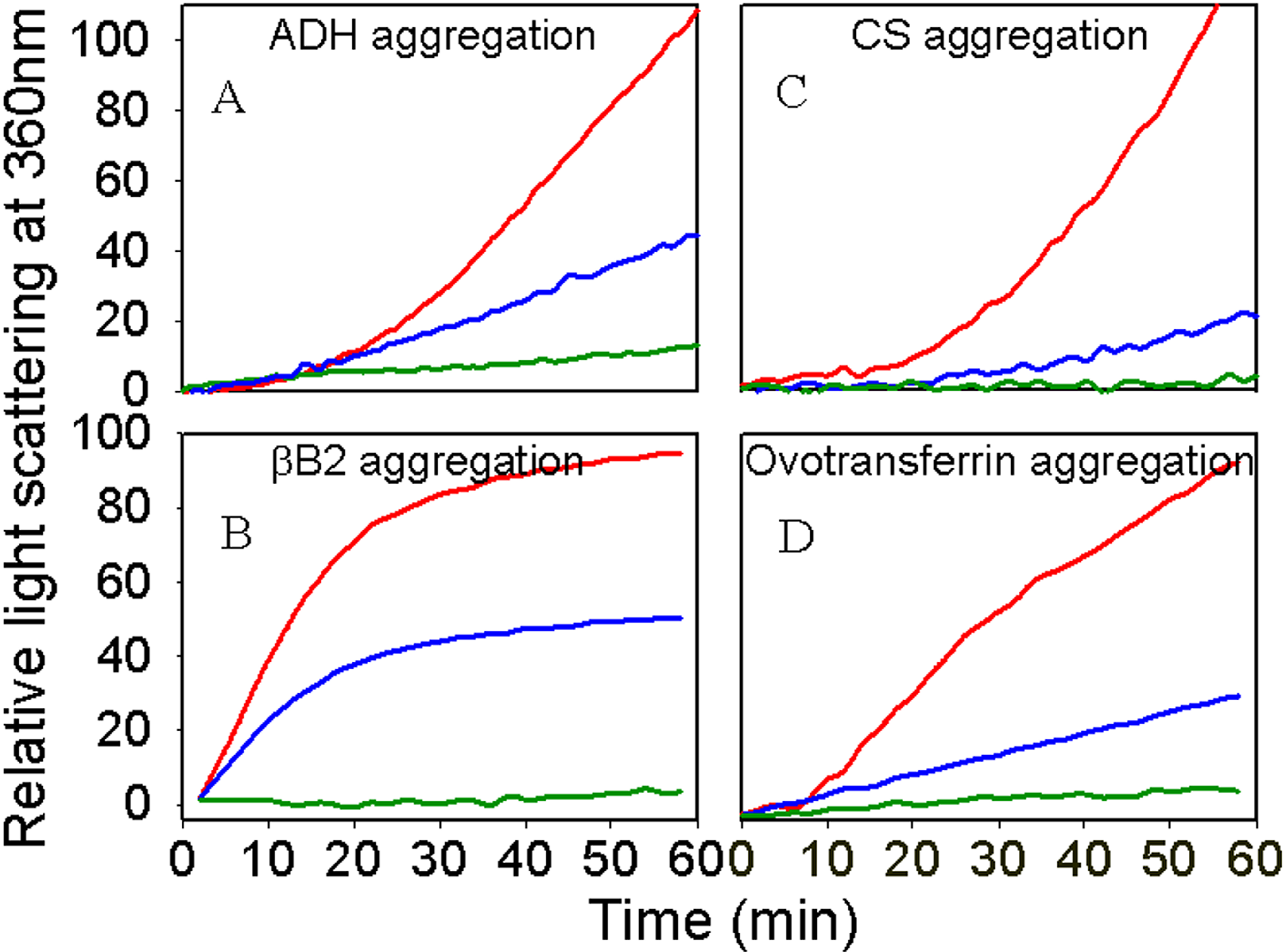
 Figure 5 of Raju, Mol Vis 2011; 17:7-15.
Figure 5 of Raju, Mol Vis 2011; 17:7-15.  Figure 5 of Raju, Mol Vis 2011; 17:7-15.
Figure 5 of Raju, Mol Vis 2011; 17:7-15. 