Figure 1. Alignment of the predicted
protein sequences of three X. laevis prominin homologs, showing
characteristic features of prominins, including the pentaspan
transmembrane topology, a conserved cysteine rich domain, conserved
leucine residues and N-glycosylation sites. We designated the three X.
laevis prominin homologs as xlProminin-1, 2, and 3, respectively.
Identical and conserved residues are indicated by differentially shaded
boxes. Predicted transmembrane domains are marked with M#. The
predicted signal peptides of xlProminin-1 and 2 are boxed. No signal
peptide was predicted for xlProminin-3. Positions of alternatively
spliced exons are indicated by arrows and the sequences of the
alternatively spliced exons are given. Note that the N- and C-termini
of the three X. laevis prominin homologs are less conserved
than
other regions. Cysteine residues at the junction of M1 and the first
intracellular domain (I1) are marked with yellow. Conserved leucine
residues of the three xlProminin homologs are marked with red.
Conserved glycosylation sites are marked with green.
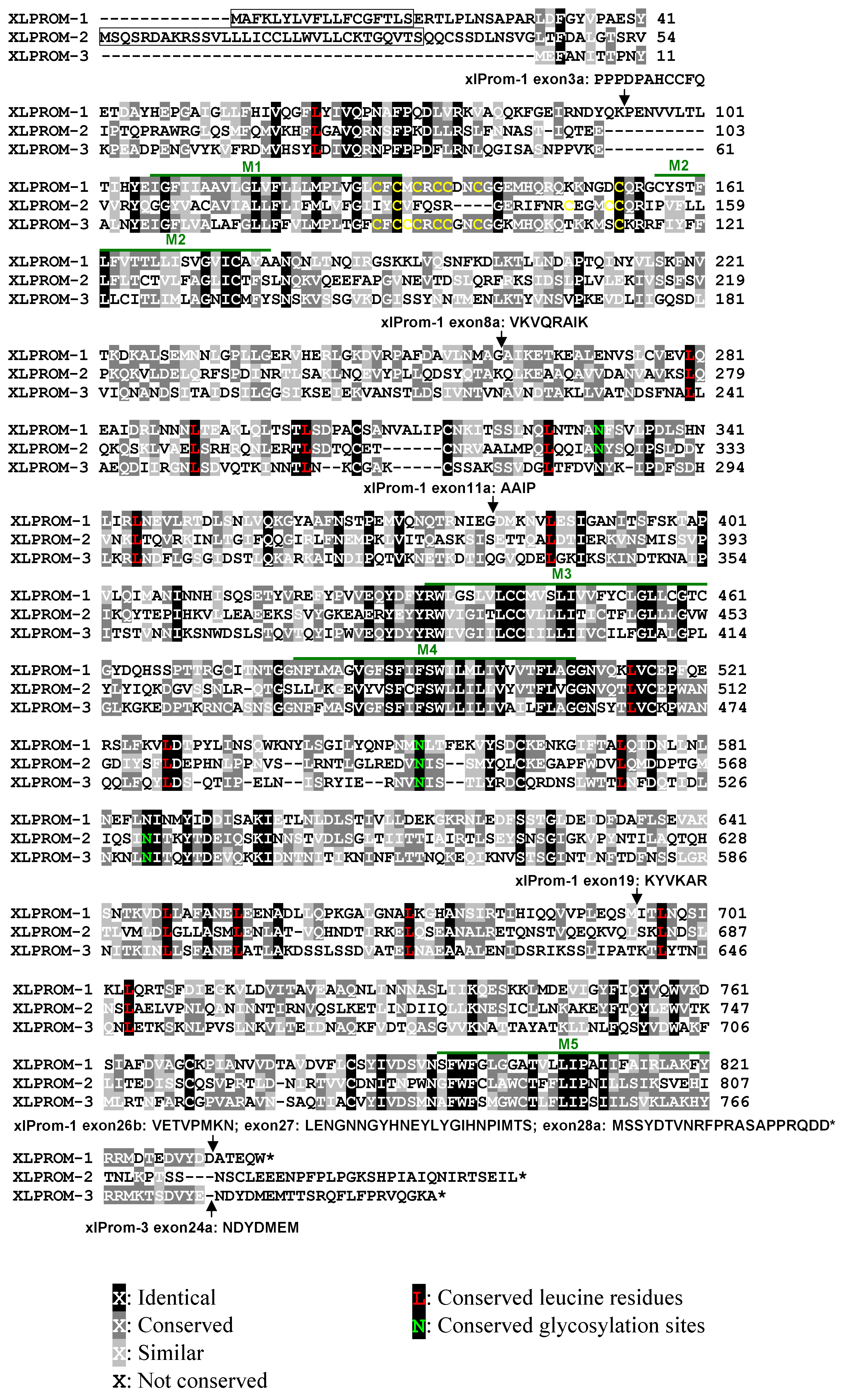
 Figure 1 of Han, Mol Vis 2011; 17:1381-1396.
Figure 1 of Han, Mol Vis 2011; 17:1381-1396.  Figure 1 of Han, Mol Vis 2011; 17:1381-1396.
Figure 1 of Han, Mol Vis 2011; 17:1381-1396. 