Figure 3. The representative tandem mass
spectra of the phosphorylated peptides 12RPFFPFHS*PSR22
and 12RPFFPFHSPS*R22. A: MS/MS spectrum
of the peptide phosphorylated at Ser-19. B: MS/MS spectrum of
the peptide phosphorylated at Ser-21. The purified phosphopeptides
samples less than 1 μg each from IMAC were first injected into a 2
cm×180 μm capillary trap column followed by LC-MS/MS and spectra
collection. Based on the tandem mass spectra of the modified peptides 12RPFFPFHS*PSR22
and 12RPFFPFHSPS*R22 as compared with the
original peptide, it can be deduced that either Ser-19 or Ser-21 is
phosphorylated. The location of the peptide fragment within the protein
is shown by the residue numbers 12 and 22 for the NH2- and
COOH-terminus of the phosphorylated peptide sequence. Identified b- and
y-ion fragment series are marked by the numbers above and under the
peptide sequence, respectively. The putative site of phosphorylation is
indicated by * and P* next to serine residues. The mass signals were
amplified fivefold, except the ion with the highest intensity.
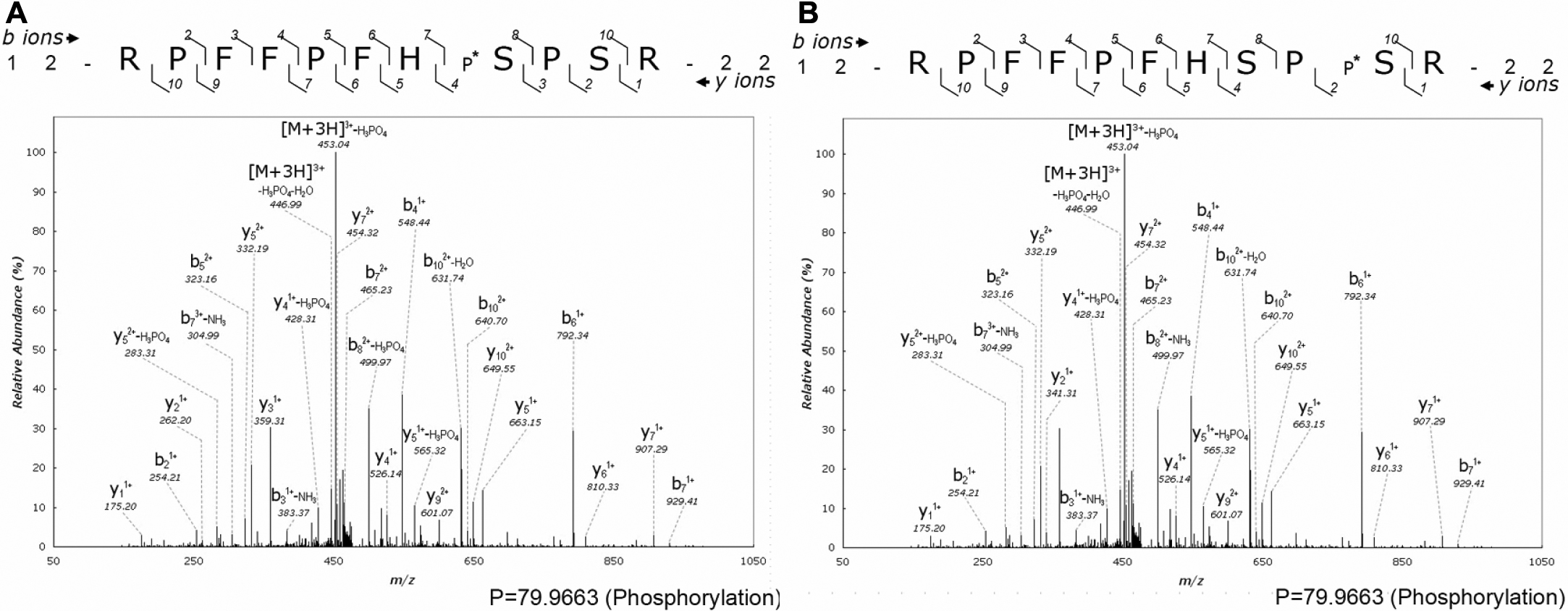
 Figure 3 of Chiou, Mol Vis 2010; 16:294-302.
Figure 3 of Chiou, Mol Vis 2010; 16:294-302.  Figure 3 of Chiou, Mol Vis 2010; 16:294-302.
Figure 3 of Chiou, Mol Vis 2010; 16:294-302. 