Figure 5. Acute bright-light stress does
not reduce the steady-state level of Ub-charged UbcM2 or the catalytic
activity of the enzyme. A: Lysates derived from the retinas of
wt mice (lanes 1 and 2) and UbcM2+/− mice (lanes 3–7)
maintained in dim light (lanes 1, 3, 4, 5) or exposed to 3,000 lux
for 6 h (lanes 2, 6, 7) were resolved by nonreducing (top blot) or
reducing (bottom blot) sodium dodecyl sulfate PAGE (SDS–PAGE) followed
by anti-UbcM2 western blotting. Ub-charged enzyme is evident as a
slower migrating band in nonreducing SDS–PAGE (top blot, indicated by
“UbcM2~Ub”). This band collapses to uncharged UbcM2 in samples exposed
to reducing agent (bottom blot). Each lane represents lysate from an
individual mouse. B: Left blot— Shown in this blot is the
enzyme used in the auto-ubiquitylation assay. The enzyme was
immunoprecipitated from UbcM2+/− retinal lysates. Each lane corresponds
to lysate from an individual mouse. Lane 1 contains antibody (Ab) only
to distinguish bands derived from the Ab versus those IPed by the Ab.
Right blot—IPed UbcM2 was combined with recombinant E1, Ub, and energy
and incubated at 37 °C for 90 min. A control lacking the
auto-ubiquitylation reaction mixture is shown in lane 14. Reaction
products were analyzed by SDS–PAGE and anti-UbcM2 western blotting.
UbcM2-Ub1 denotes a band that corresponds to
mono-ubiquitylated UbcM2, and UbcM2-Ub2 denotes a band that
corresponds to two Ub molecules conjugated to the enzyme. Vertical
lines between lanes 1 and 2 (left blot) and lanes 14 and 15 (right
blot) indicate lanes were not adjacent on original blots. Molecular
weight markers were run between samples 1 and 2 and samples 14 and 15.
The migration of heavy chain is indicated, and the migration of
molecular weight markers is shown on the left for (A) and (B).
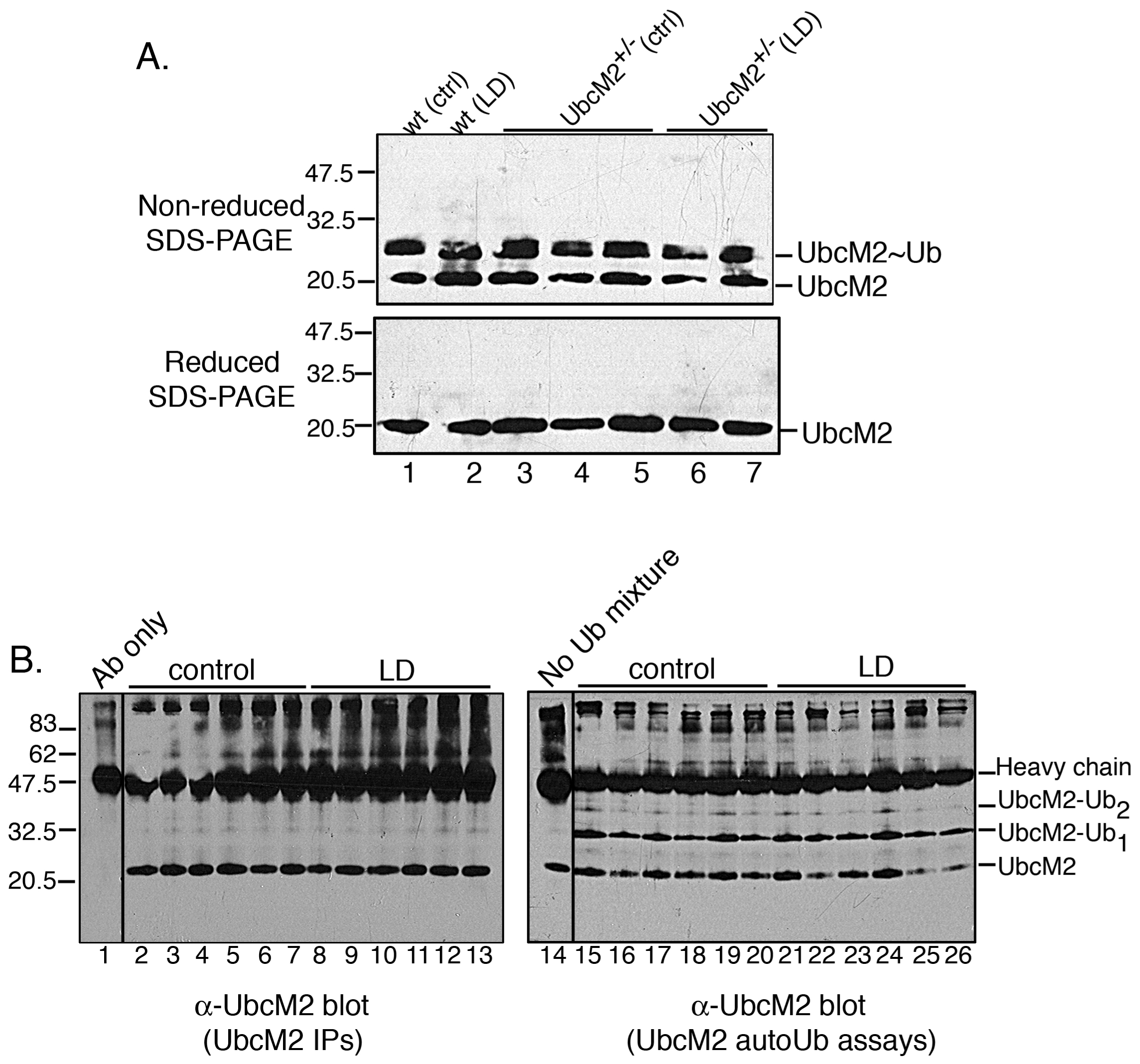
 Figure 5 of Mirza, Mol Vis 2010; 16:2425-2437.
Figure 5 of Mirza, Mol Vis 2010; 16:2425-2437.  Figure 5 of Mirza, Mol Vis 2010; 16:2425-2437.
Figure 5 of Mirza, Mol Vis 2010; 16:2425-2437. 