Figure 1. Characterization of the
sensitivity and specificity of class III ubiquitin conjugating enzyme
(E2) antibodies. A: Dot blot assay using recombinant
glutathione S-transferase (GST) or GST-E2 fusion proteins. The
indicated recombinant proteins (10, 1, or 0.1 ng) were spotted on
pieces of nitrocellulose paper in quintuplicate. The blots were blocked
in 5% milk/TBST and then incubated with anti-GST, anti-UbcM3,
anti-UBE2E2, anti-UbcM2, or a commercial antibody against human UbcM3
(anti-UbcH6). To the right of the blots is a diagram of the class III
E2s highlighting the relative location of the conserved catalytic core
domain (UBC), the number of residues in each protein, and the residue
corresponding to the end of the unique N-terminal extension. B:
Mouse retinal lysate (10 or 30 μg) was resolved by sodium dodecyl
sulfate PAGE (SDS–PAGE) in quadruplicate, transferred to
nitrocellulose, and probed with the indicated antibodies. The migration
of UbcM2 and UbcM3 is indicated to the right of the anti-UbcH6 blot.
Two distinct isoforms of UbcM3 are detected (arrows). The migration of
molecular weight markers is indicated on the left. C: siRNA
experiments in HeLa cells to demonstrate that targeted knockdown of
UbcM2 results in loss of the band denoted as UbcM2 but does not affect
UbcM3 expression (left blot), and targeted knockdown of UbcM3 results
in loss of detection of both isoforms of the enzyme (right blot). The
migration of molecular weight markers is indicated to the left of the
blots.
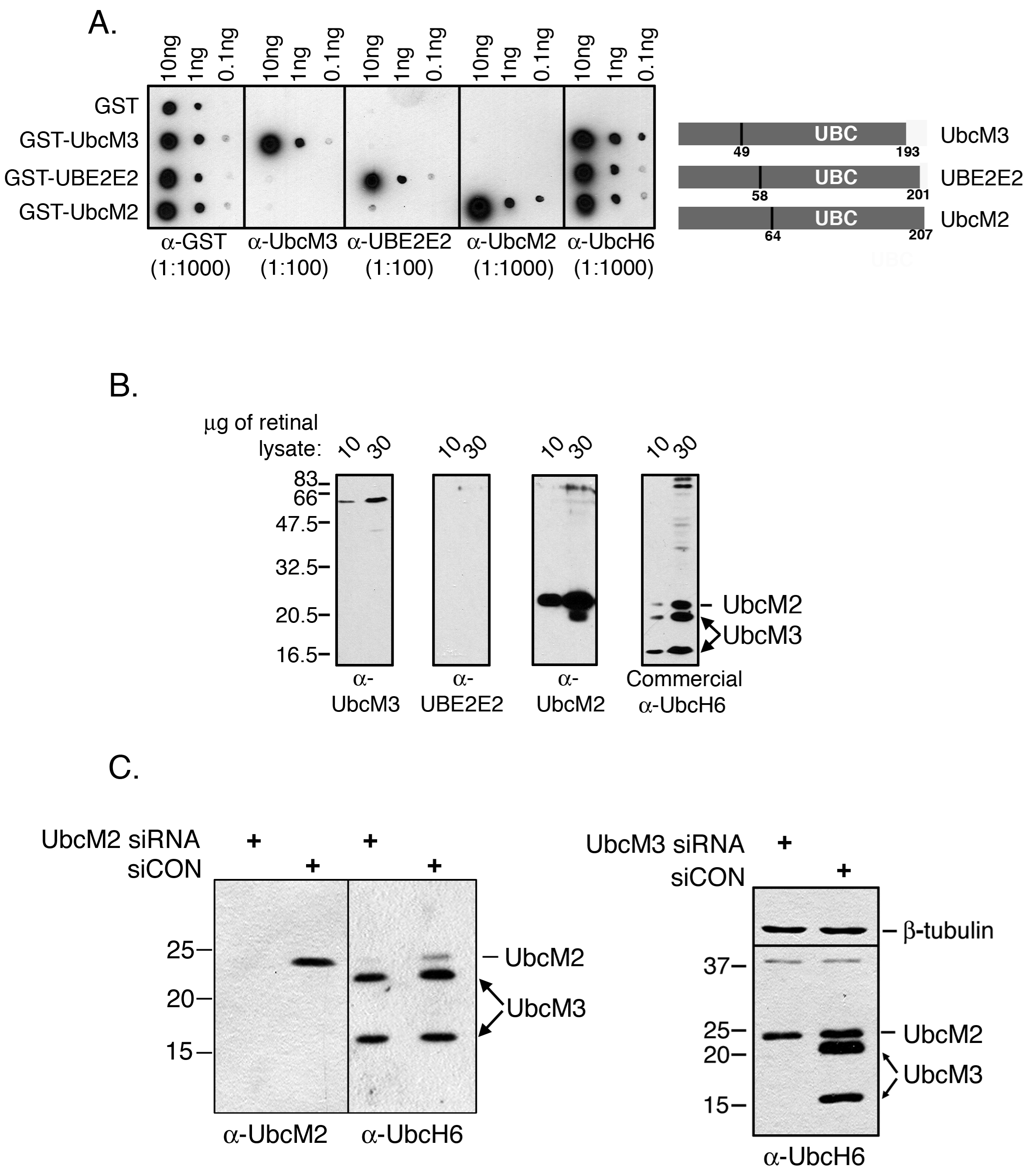
 Figure 1 of Mirza, Mol Vis 2010; 16:2425-2437.
Figure 1 of Mirza, Mol Vis 2010; 16:2425-2437.  Figure 1 of Mirza, Mol Vis 2010; 16:2425-2437.
Figure 1 of Mirza, Mol Vis 2010; 16:2425-2437. 