Figure 6. SDS–PAGE analysis and
fluorescence determination to examine binding of FFCK to untruncated
βA3-crystallin and its two major truncated products. Lanes 4 and 5
represents βA3-crystallin truncated species, which were recovered
following treatment of βA3-crystallin with CHAPS, and were labeled with
FFCK. The lanes 6 and 7 represented βA3-crystallin treated with CHAPS
but complete degradation of intact crystallin to 22 and 27 kDa
species was not allowed to determine if all three species (intact, 22,
and 27 kDa species) show labeling with FFCK. The untruncated
βA3-crystallin (Coomassie blue stained in lane 7 of A) and its
two truncated species of 22 and 27 kDa (Coomassie blue-stained
bands in lane 4 and 7 in A) showed binding to FFCK (25 μM,
final concentration) as represented by fluorescent bands (lane 4 and 7
in B). The results suggest that because of FFCK binding, the
protease active site is present in both untruncated βA3-crystallin and
its major truncated species II and III.
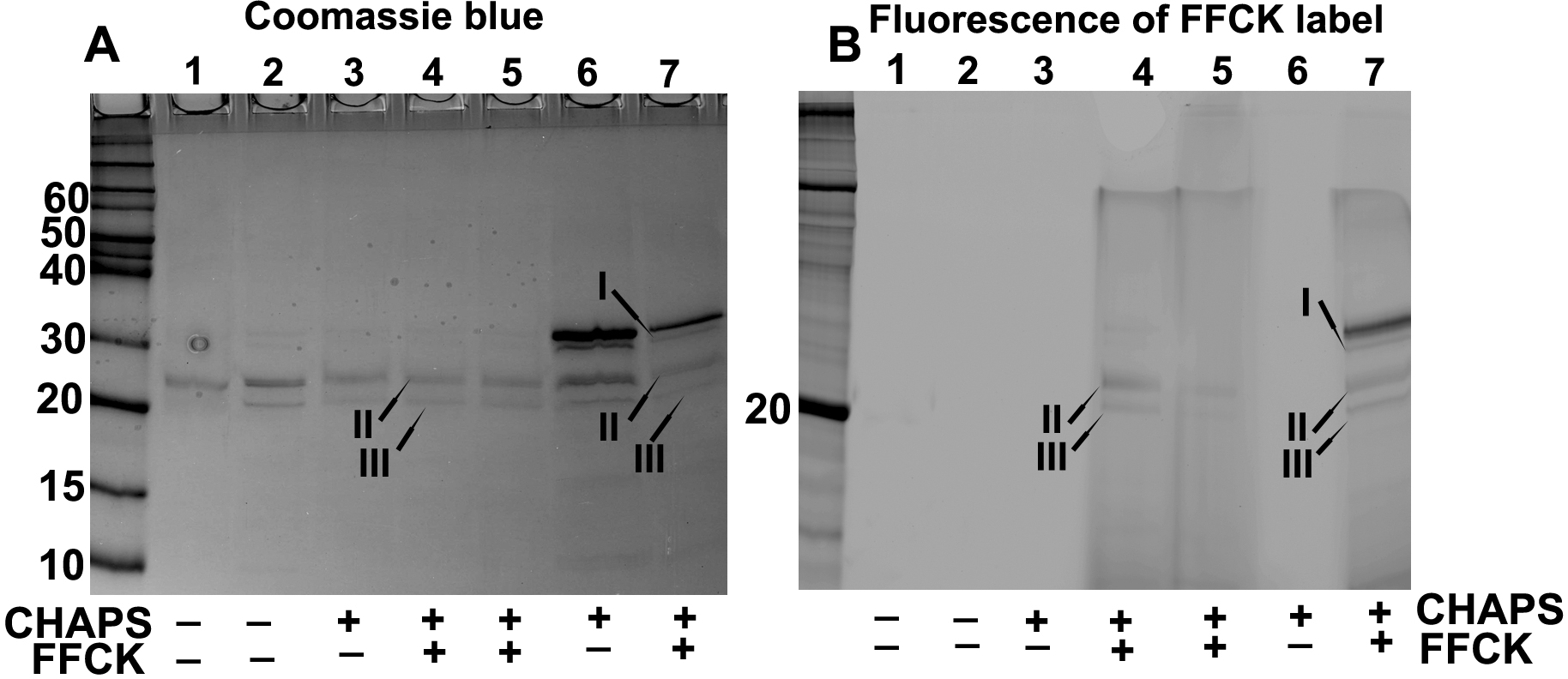
 Figure 6 of Gupta, Mol Vis 2010; 16:2242-2252.
Figure 6 of Gupta, Mol Vis 2010; 16:2242-2252.  Figure 6 of Gupta, Mol Vis 2010; 16:2242-2252.
Figure 6 of Gupta, Mol Vis 2010; 16:2242-2252. 