Figure 5. Inhibition of autodegrdation of
βA3-crystalllin by protease inhibitors in the presence of CHAPS. The
criterion for inhibition in the study was whether an inhibitor was able
to prevent signature autodegradation of βA3-crystallin (50 μg, used
with each inhibitor) into the three truncated specific I, II, and III.
The inhibitors used in the study are identified at the top of the gel.
Only the inhibitors of serine-type proteases such as phenylmethyl
sulfonyl fluoride (PMSF, 2 mM, lane 2), aprotinin (25 μg/ml, lane 4)
and chymostatin (lane 11) inhibited autodegradation of the crystallin,
whereas 4(2-aminoehtyl)-benzene sulfonyl fluoride (2 mM, lane 3) showed
partial inhibition of autodegradation. In contrast, the
cysteine-protease inhibitors (E-64, 100 μM [lane 5], N-ethylmaleimide,
5 mM [lane 6] and iodoacetamide, 5 mM [lane 7], and metallo-proteinase
inhibitors (EDTA, 5 mM [EDTA, lane 8] and ethylene glycotetraacetic
acid, 5 mM [EGTA, lane 9] did not stop autodegradation of the
crystallin.
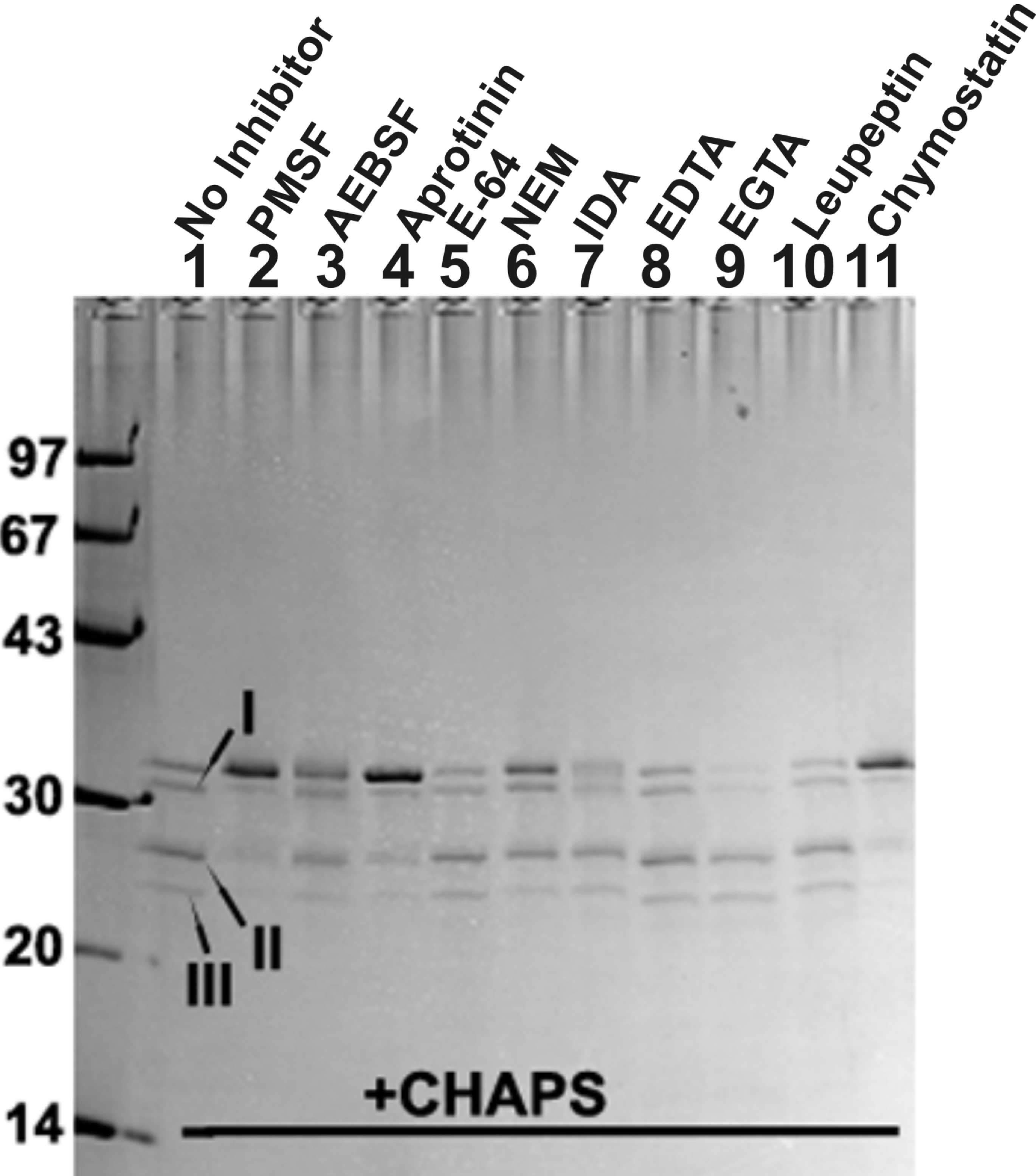
 Figure 5 of Gupta, Mol Vis 2010; 16:2242-2252.
Figure 5 of Gupta, Mol Vis 2010; 16:2242-2252.  Figure 5 of Gupta, Mol Vis 2010; 16:2242-2252.
Figure 5 of Gupta, Mol Vis 2010; 16:2242-2252. 