Figure 1. Purification of recombinant
βA3-crystallin by the nickel-affinity column chromatographic method.
During purification of βA3-crystallin from the soluble protein fraction
of the E. coli cell lysate, the bound proteins were eluted
using 250 mM imidazole, and each fraction was analyzed using a 15%
polyacrylamide gel using the SDS–PAGE method. Fractions (lanes 1 to 9)
containing the single major protein band of βA3-crystallin were pooled,
dialyzed, and used for further experiments. Circled bands 1, 2, and 3
were excised from gels, trypsin-digested and analyzed by quadruple ion
trap (Q-TRAP) mass spectrometric method. The analysis identified these
circled species (1, 2, and 3) as βA3-crystallin suggesting that the
parent crystallin was partially degraded to produce two minor
crystallin fragments during purification.
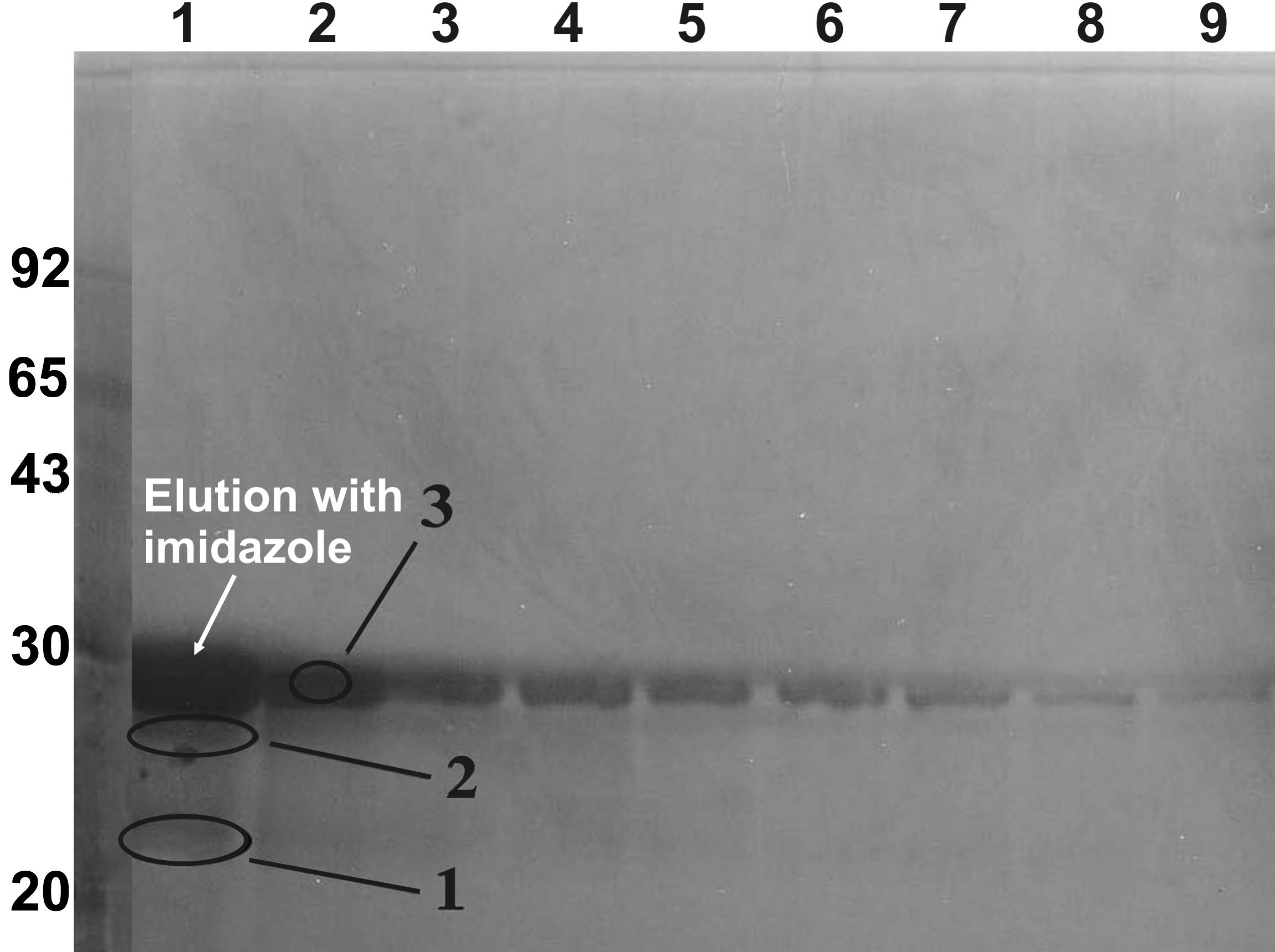
 Figure 1 of Gupta, Mol Vis 2010; 16:2242-2252.
Figure 1 of Gupta, Mol Vis 2010; 16:2242-2252.  Figure 1 of Gupta, Mol Vis 2010; 16:2242-2252.
Figure 1 of Gupta, Mol Vis 2010; 16:2242-2252. 