Figure 3. Pb alters α-crystallin processing in cultured lenses. A: Two dimensional gels of HPLC purified α-crystallin from cultured lenses (3 days; 1 uM Pb(NO3)2). B: Quantification of the relative amounts of various post-translationally modified forms of α-crystallin in cultured lenses
(3 days; 1 uM Pb(NO3)2). Spot densities (normalized to either dominant αA- or αB-crystallin protein spot) of different isoforms of α-crystallin
are altered with Pb-exposure (3 days; 1 uM Pb(NO3)2). Only protein spots sufficiently resolved from neighboring protein spots were quantified. C: α-Crystallin purified from lenses exposed in vitro to Pb (3 days; 1 uM Pb(NO3)2) does not exhibit alterations in chaperone function. Dash/dot line (- - -) shows aggregation of denatured lactalbumin without
α-crystallin. Solid line (—) shows aggregation of denatured lactalbumin in the presence of α-crystallin purified from lenses
cultured in control media. Unconnected circles (o o o) show aggregation of denatured lactalbumin in the presence of α-crystallin
purified from lenses cultured in the presence of Pb.
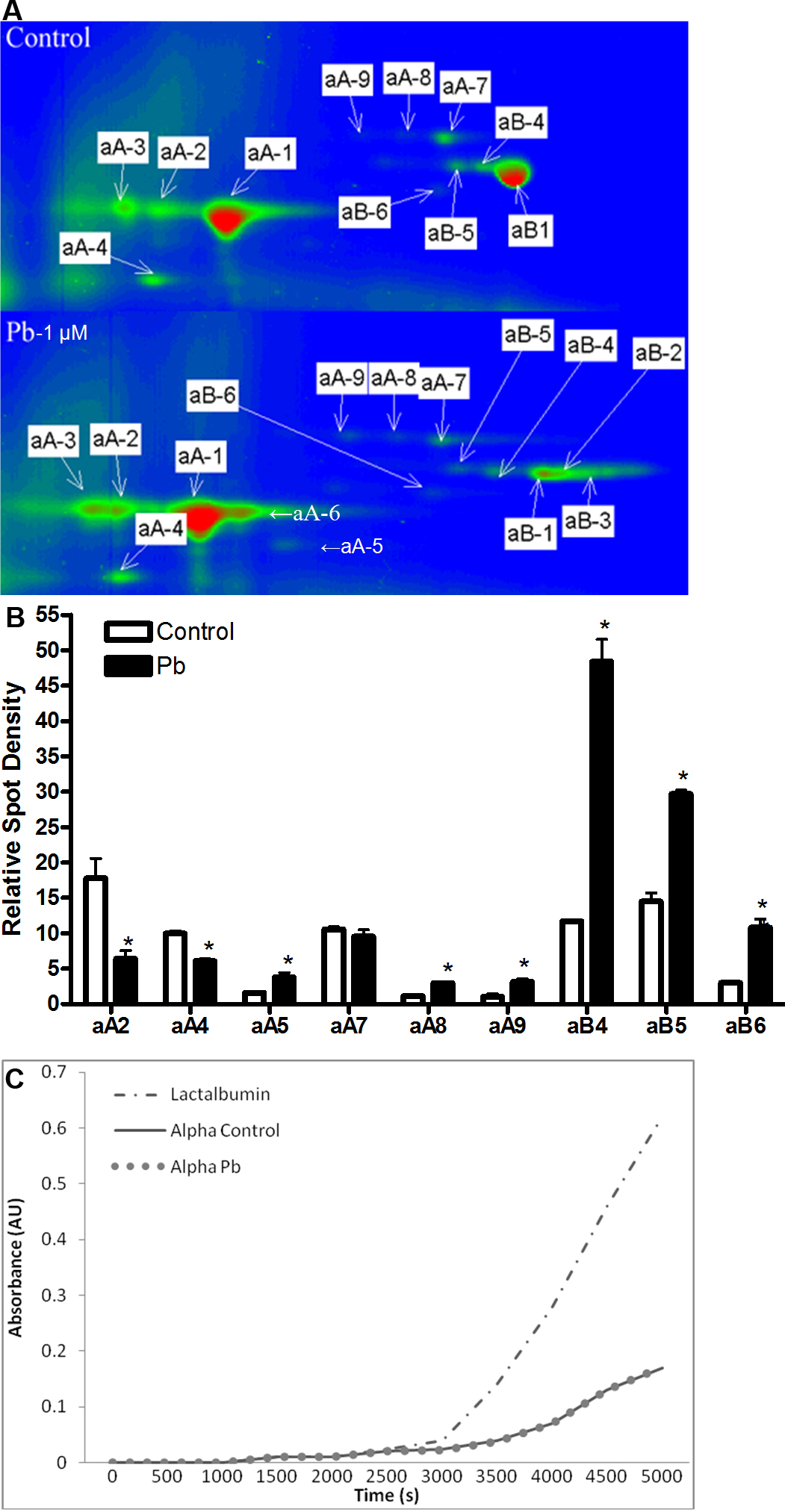
 Figure 3 of
Neal, Mol Vis 2010; 16:2137-2145.
Figure 3 of
Neal, Mol Vis 2010; 16:2137-2145.  Figure 3 of
Neal, Mol Vis 2010; 16:2137-2145.
Figure 3 of
Neal, Mol Vis 2010; 16:2137-2145. 