Figure 4. ADAM9 mutation in canine
cone-rod dystrophy 3 (crd3) affected dogs. A schematic drawing, of part
of the canine ADAM9 genomic sequence, aligning the normal and
crd3 mutant alleles suggests a possible mutation mechanism. Bordered
square boxes represent exons 14–17, lines between are introns;
unbordered rectangular boxes represent long-terminal repeat sequences
(LTR). Within the LTR are the nucleotides identified as single
nucleotide polymorphisms (SNPs). A suggested mechanism of unbalanced
recombination is illustrated, resulting in the deletion of part of
intron 14, all of exon 15, intron 15, exon 16, and part of intron 16,
as well as the formation of a single chimeric LTR. Arrows represent the
location of primers used to identify the mutation. Primer pair F29/R31
amplifies the mutant allele and results in a 1,515 bp PCR product from
genomic DNA. Primer pair F10/R10 amplifies the normal allele and
results in a 602 bp PCR product from genomic DNA. B: Gel
electrophoresis of multiplex PCR reaction identifies ADAM9 alleles from
normal (N), crd3-carrier (C), and crd3-affected (A) dogs. The lower,
602 bp band is the normal allele, amplified by primer pair F10/R10
located within intron 15, which is deleted in the affected allele. The
upper 1,515 bp band is the mutant allele, amplified by primer pair
F29/R31, which flanks the >23 kb sequence deleted in the affecteds.
Both bands are present in the heterozygous carrier dog. The normal and
crd3 mutant canine ADAM9 transcripts, and their corresponding
predicted translation products, are aligned (C) to illustrate
their differences schematically. The protein domains represented are
those predicted by Swiss-Prot for the human ADAM9 protein. Exons 15 and
16 are missing from the mutant transcript, and a premature stop codon
is introduced (arrow). The mutant protein translated from this
transcript is predicted to be truncated, lacking the last 287 amino
acids of the C-terminus, part of the cysteine-rich domain, the complete
epidermal growth factor (EGF)-like domain, the transmembrane domain,
and the cytoplasmic tail.
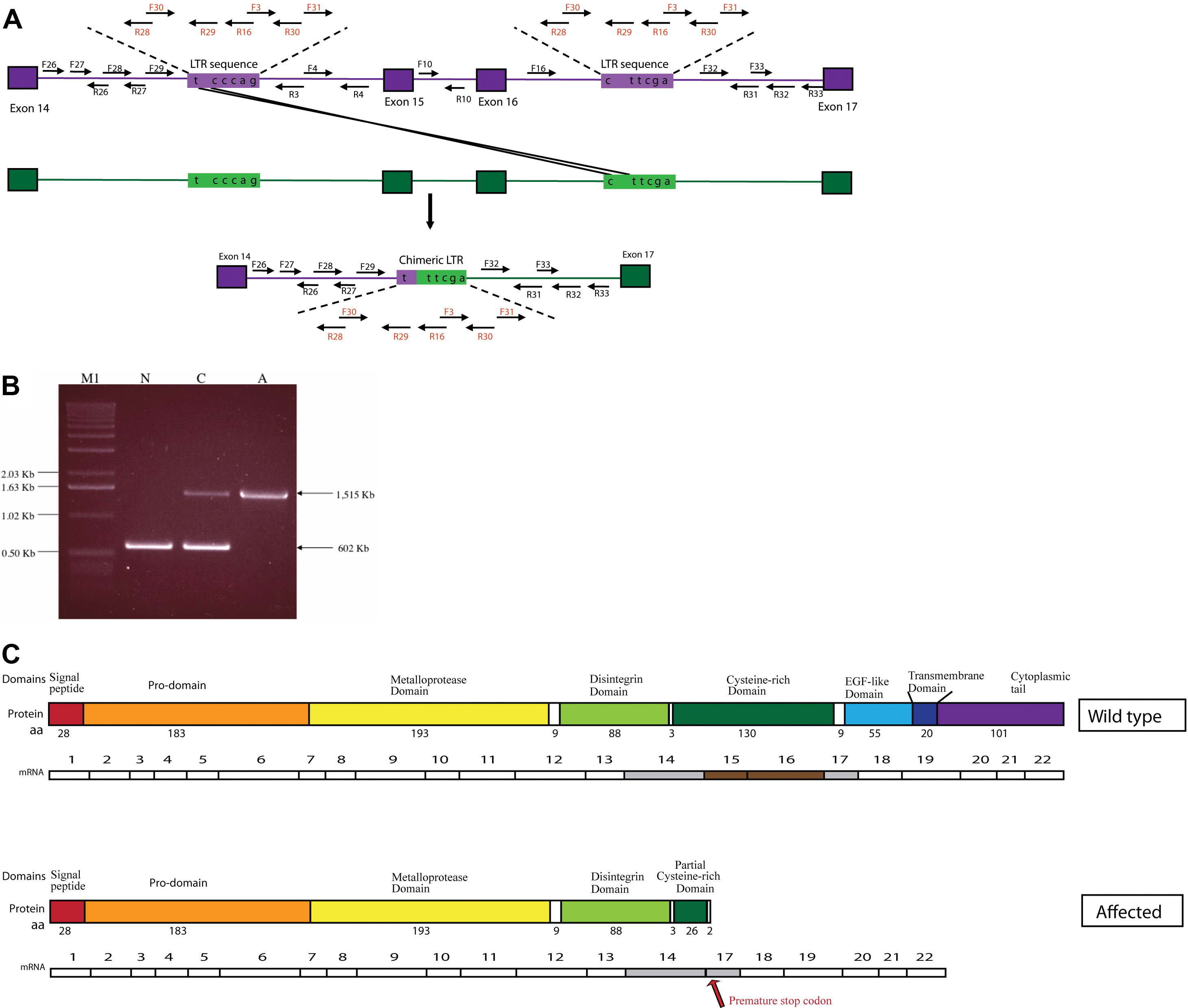
 Figure 4 of Goldstein, Mol Vis 2010; 16:1549-1569.
Figure 4 of Goldstein, Mol Vis 2010; 16:1549-1569.  Figure 4 of Goldstein, Mol Vis 2010; 16:1549-1569.
Figure 4 of Goldstein, Mol Vis 2010; 16:1549-1569. 