Figure 5. Effect of protein kinase
inhibitors on phosphorylation of LRP-1. Retinal ganglion cell (RGC)-5
cells were left untreated or treated for 24 h with staurosporine (SS;
2.0 μM), norrin (25 ng/ml), with or without H-89 (n=3 experiments). At
the end of 24 h, proteins were extracted and immunoprecipitated by
using antibodies against lipoprotein-related receptor-1 (LRP-1).
Immunoprecipitated proteins were then subjected to western blot
analysis by using antibodies against phosphoserine, phosphotyrosine,
and LRP-1 (A). Relative amount of proteins was determined by
densitometric analysis (B). LRP-1 was expressed constitutively
in norrin-treated cells and its expression did not change regardless of
treatment condition (A). LRP-1 was phosphorylated at
Tyr-residues constitutively in norrin-treated cells and phosphorylation
status of LRP-1 at Tyr-residues did not change with any of the
treatment condition (A). In addition, LPR-1 was constitutively
phosphorylated at Ser-residues in norrin-treated cells (A).
Compared to Ser-phosphorylation of LRP-1 in norrin-treated cells,
Ser-phosphorylation of LRP-1 was significantly reduced when cells were
treated with H-89 (A, B, *p<0.05). In addition, compared to
Ser-phosphorylation of LRP-1 under SS and norrin-treated conditions,
Ser-phosphorylation of LRP-1 was further reduced under H-89, SS, and
norrin-treated conditions (A,B, **p<0.05). Cell viability
assays indicate that, in the absence of SS, lower concentrations of
H-89 had no effect on cell survival, but higher concentrations of H-89
(2.5–10.0 μM) decreased cell survival significantly regardless of
norrin's presence (C, *p<0.05). Furthermore, in the presence
of SS, lower concentrations of H-89 had no effect on cell survival, but
higher concentrations of H-89 (2.5–10.0 μM) decreased norrin-mediated
cell survival (D, *p<0.05).
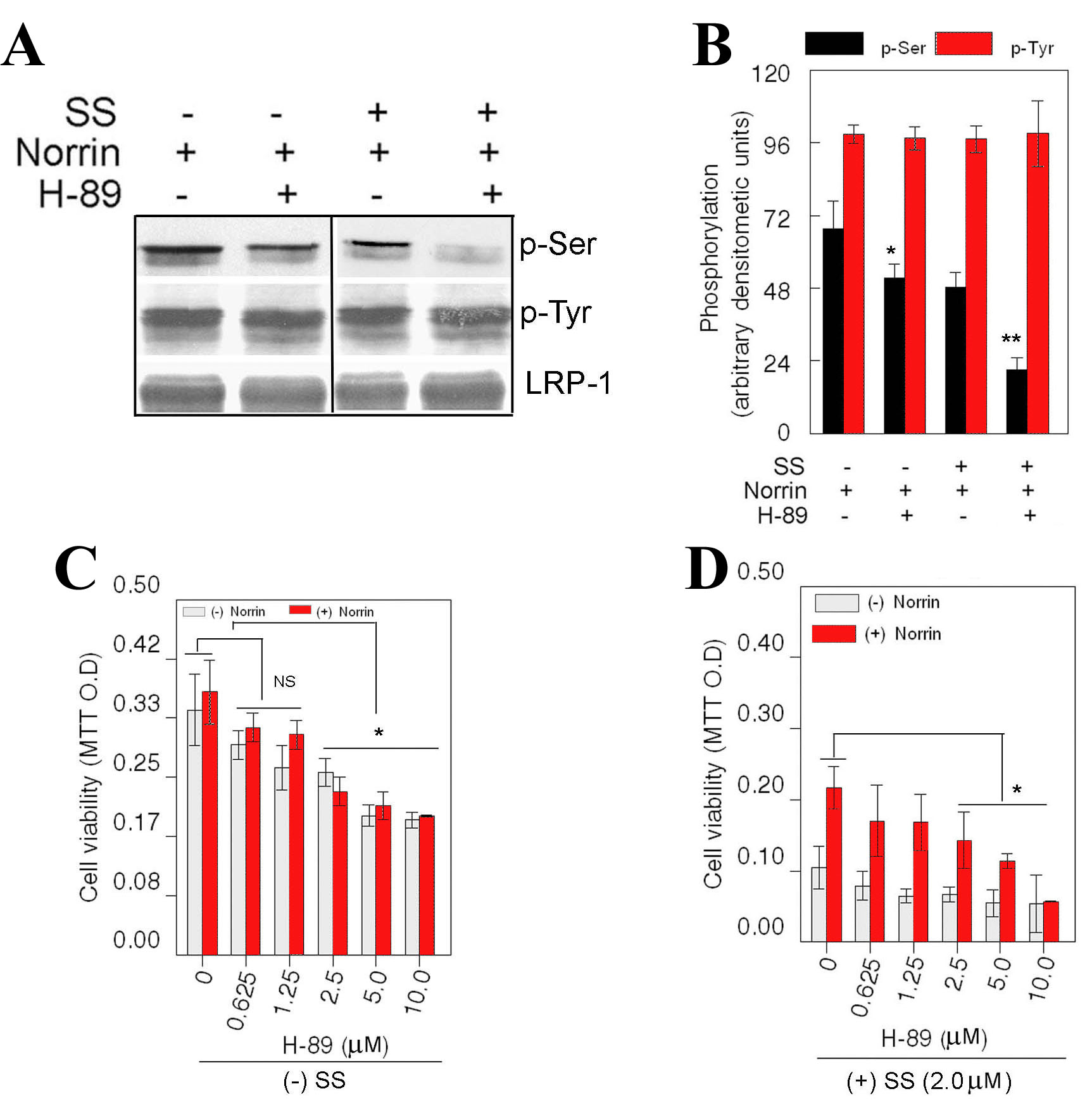
 Figure 5 of Lin, Mol Vis 2009; 15:26-37.
Figure 5 of Lin, Mol Vis 2009; 15:26-37.  Figure 5 of Lin, Mol Vis 2009; 15:26-37.
Figure 5 of Lin, Mol Vis 2009; 15:26-37. 