Figure 2. Intrinsic tryptophan
fluorescence was used to investigate the quenching and denaturation of
transforming growth factor beta-induced protein (TGFBIp) A:
Fluorescence traces of TGFBIp (0.1 mg/ml) in guanidine hydrochloride
(GndHCl), ranging from 0 to 6 M, were shown. Samples were excited at
295 nm and scanned for the tryptophan emission. Inset: Fluorescence
quenching experiments by acrylamide and KI. B: The emission
maximum (Emax) and the intensity-averaged emission maximum
(IAEM) were obtained by plotting the intrinsic tryptophan fluorescence
traces versus GndHCl concentrations. Inset: Denaturation experiments by
urea at various concentrations. Plot of the shift of emission maximum
versus urea concentration clearly shows that urea fails to unfold
TGFBIp, even up to 8 M. C: Thioflavin T (ThT) fluorescence
intensity at 485 nm was used to determine the fibril formation of
TGFBIp at various GndHCl concentrations (n=3, bar=S.D.).
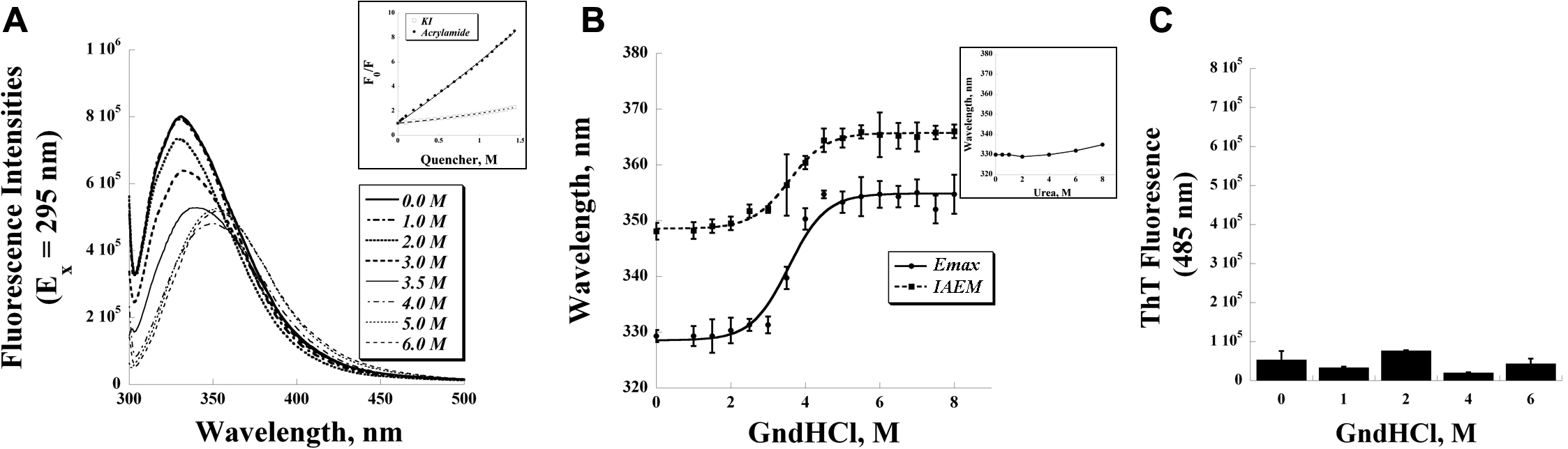
 Figure 2 of Grothe, Mol Vis 2009; 15:2617-2626.
Figure 2 of Grothe, Mol Vis 2009; 15:2617-2626.  Figure 2 of Grothe, Mol Vis 2009; 15:2617-2626.
Figure 2 of Grothe, Mol Vis 2009; 15:2617-2626. 