Figure 10. Mel1a and Mel1c
immunocytochemistry of whole-mounted Xenopus laevis surface
corneal epithelium obtained at 4-h intervals during a 24-h light–dark
cycle. Frogs were housed under a 12 h:12 h light–dark cycle (6:00 AM:
lights on; 6:00 PM: lights off). All tissues in this figure were
obtained in the dark. Mel1c labeling is represented in green (A,
D, and G) and Mel1a labeling is represented in red (B,
E, and H). The yellow labeling in the merged images (C,
F, and I) indicates regions of co-localization of the
red and green signal. A-C: Corneas obtained at 8:00 PM (2 h
after lights off). Mel1c immunolabeling is almost exclusively located
in the cytoplasm, but there is intense Mel1a immunoreactivity present
in the lateral membranes, and also in the cytoplasm. The cytoplasmic
immunolabeling of Mel1a and Mel1c is not co-localized. D-F:
Corneas obtained at 12:00 M (mid-dark). Most of the Mel1c
immunoreactivity is in the cytoplasm, although some lateral membrane
labeling is also detected. Mel1a immunoreactivity is predominant in the
lateral membranes, but there are many irregular-appearing cytoplasmic
compartments that express Mel1a immunoreactivity, and they do not
co-localize with the Mel1c cytoplasmic labeling. Essentially, all Mel1c
lateral membrane labeling is co-localized with Mel1a membrane labeling.
G-I: Corneas obtained at 4:00 AM (2 h before lights on).
Most of the Mel1c immunoreactivity is located in the cytoplasm, with
very little membrane labeling detected. Most of the Mel1a
immunoreactivity is located on the lateral membranes, with some
immunoreactivity also appearing in irregular cytoplasmic compartments.
The Mel1a and Mel1c cytoplasmic labeling is not co-localized. Nuclei
are stained with DAPI. The confocal images in all panels are comprised
of three optical slices of 400 nm each in the z-series. The
magnification bar (I) represents 20 µm.
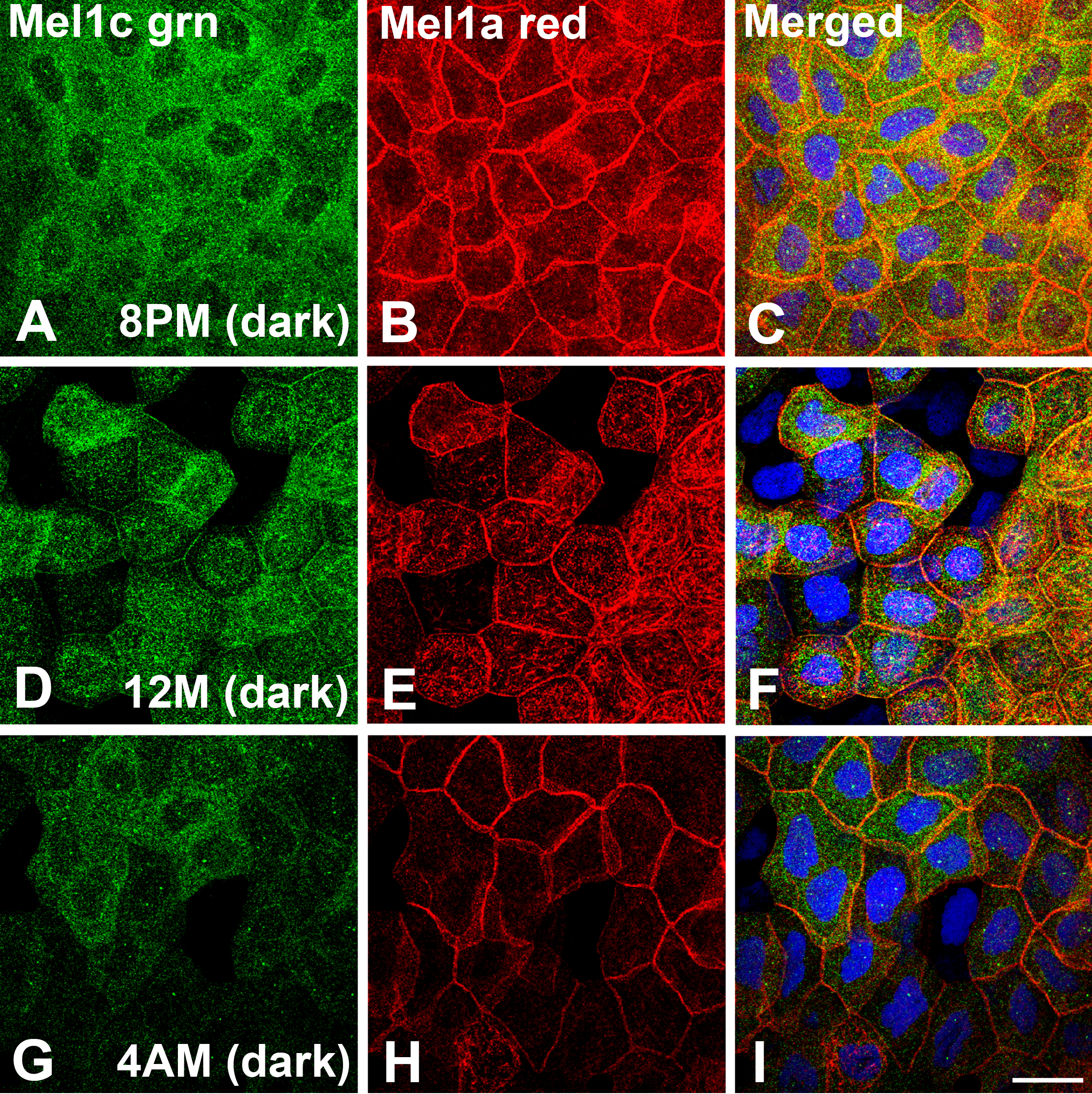
 Figure 10 of Wiechmann, Mol Vis 2009; 15:2384-2403.
Figure 10 of Wiechmann, Mol Vis 2009; 15:2384-2403.  Figure 10 of Wiechmann, Mol Vis 2009; 15:2384-2403.
Figure 10 of Wiechmann, Mol Vis 2009; 15:2384-2403. 