Figure 7. Thermal stability of wild type
and G98R αA-crystallin upon Cu2+-binding. Aggregation of 0.2
mg/ml of αA-crystallin (curve 1) and G98R αA-crystallin (curve 2) in
buffer B and of 30 μM Cu2+-treated samples of αA-crystallin
(curve 3) and G98R αA-crystallin (curve 4) is shown. The aggregation
was monitored by light scattering at 465 nm as a function of
temperature. G98R mutation in αA-crystallin leads to decreased thermal
stability upon Cu2+-binding.
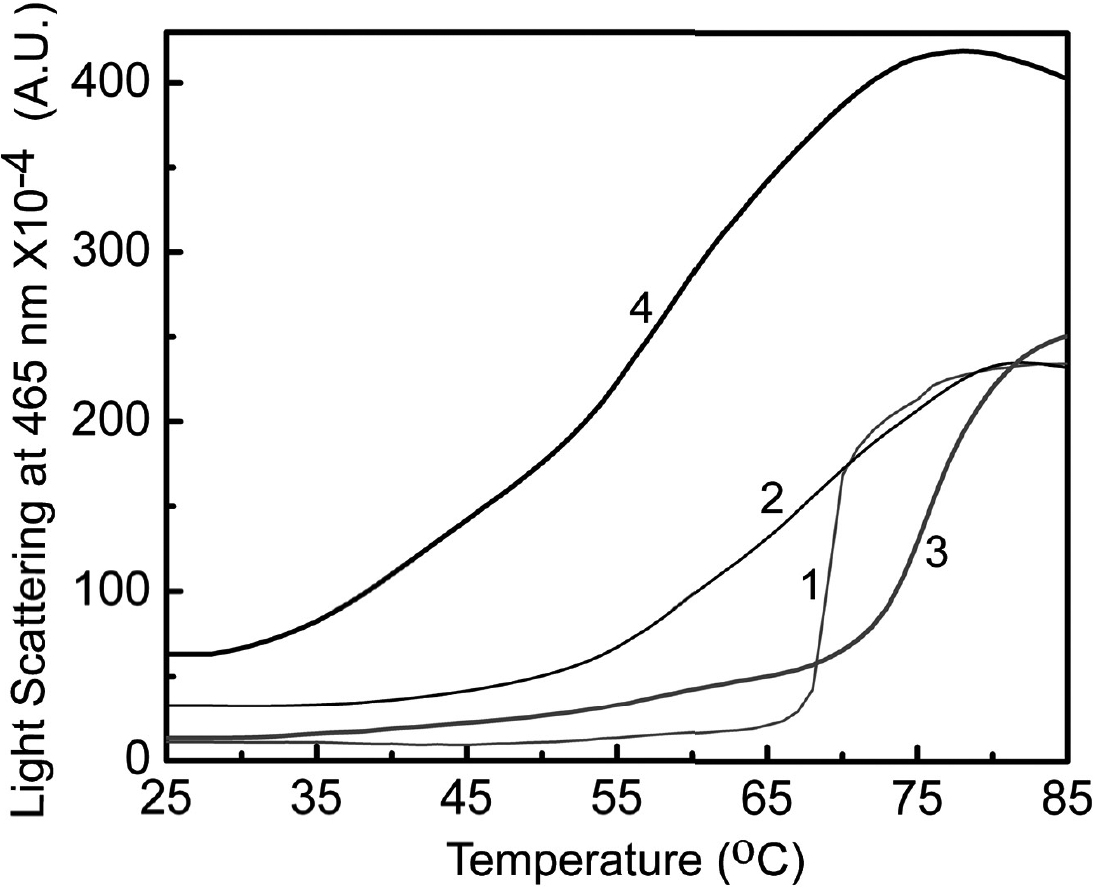
 Figure 7 of Singh, Mol Vis 2009; 15:2050-2060.
Figure 7 of Singh, Mol Vis 2009; 15:2050-2060.  Figure 7 of Singh, Mol Vis 2009; 15:2050-2060.
Figure 7 of Singh, Mol Vis 2009; 15:2050-2060. 