Figure 1. Quenching of intrinsic
fluorescence upon binding of Cu2+. A:
Intrinsic tryptophan fluorescence spectra of 0.1 mg/ml sample of
αA-crystallin in buffer A at indicated concentrations (in µM) of Cu2+
are shown. B: The extent of fluorescence quenching [(F0-F)/F0]
of 0.1 mg/ml αA- (○) and G98RαA-crystallin (●) at 25 °C is shown as a
function of Cu2+ concentration. The extent of
fluorescence quenching of the controls, 5 μM NATA (■), 0.1 mg/ml of
thyroglobulin (△) and α-synuclein (▼) as a function of Cu2+
concentration are also shown. F0 and F are the
fluorescence intensities at 337 nm in the absence and in the presence Cu2+.
In the case of α-synuclein which lacks tryptophan residue, fluorescence
intensity of tyrosine residues was measured at 300 nm. Both αA- and
G98RαA-crystallin exhibit similar extent of fluorescence quenching
indicating that they have similar Cu2+-binding properties.
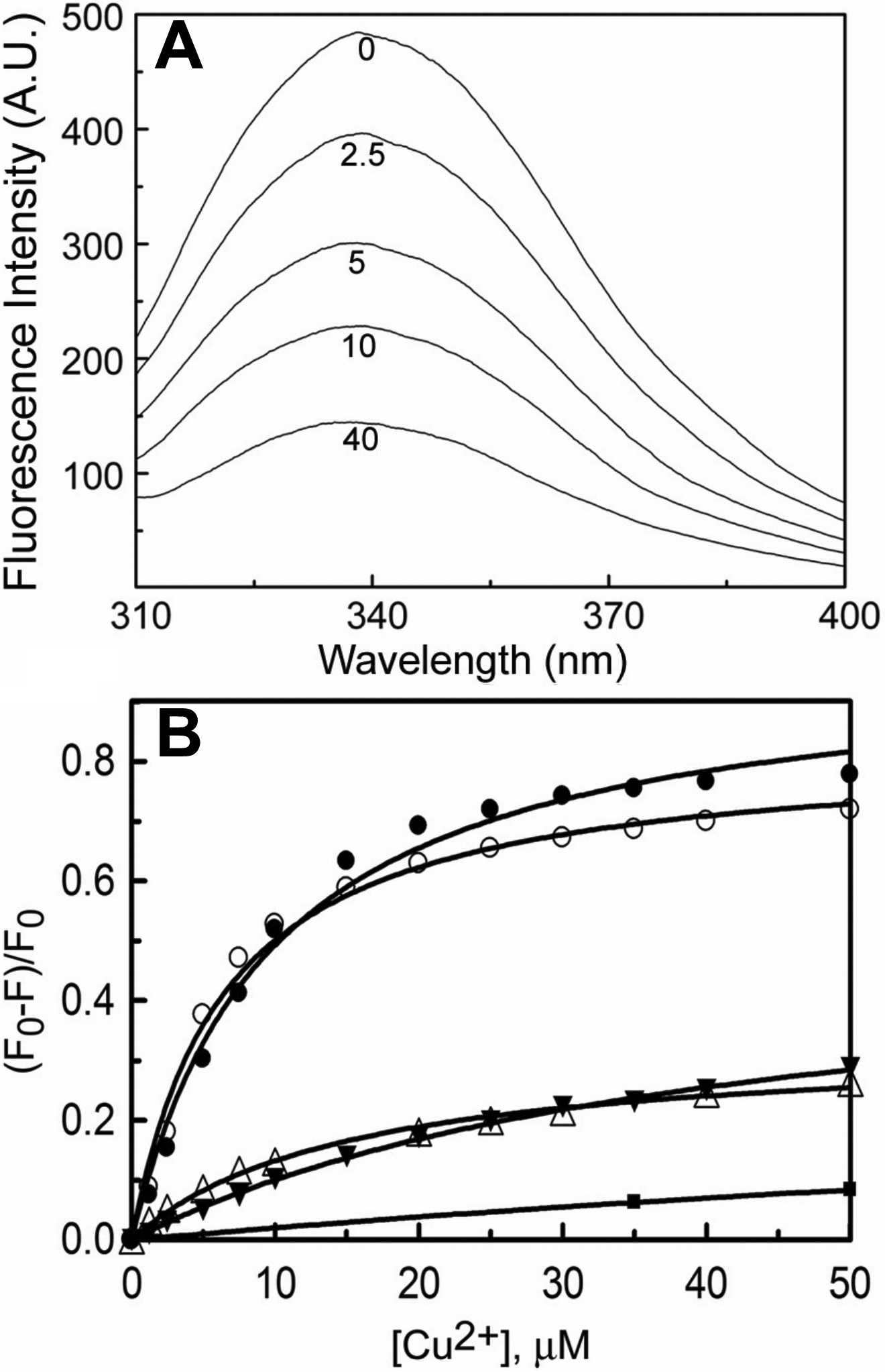
 Figure 1 of Singh, Mol Vis 2009; 15:2050-2060.
Figure 1 of Singh, Mol Vis 2009; 15:2050-2060.  Figure 1 of Singh, Mol Vis 2009; 15:2050-2060.
Figure 1 of Singh, Mol Vis 2009; 15:2050-2060. 