Figure 6. Thermal stabilities of wild-type αB-crystallin and its mutants. The turbidity change after heating was measured at 360 nm.
Each sample (0.5 ml) of the same concentration (36 μM) was heated from 20 °C to 80 °C. Wild-type αB-crystallin and Δ11 are
susceptible to aggregation and show serious light scattering (turbidity) at about 62 °C. Mutants Δ2, Δ5, and Δ7 show protein
aggregation when heated to 70 °C. Mutant Δ1 becomes turbid near 67 °C and Δ10 is also susceptible to aggregation when heated
to 67 °C, which displays greater light scattering than Δ2, Δ5, and Δ7. Mutant Δ12 is least stable as compared to other mutants
and susceptible to aggregation at 57 °C. B: Conformational change of crystallin unfolding with heating. The molar ellipticity change at 217 nm of far-UV CD spectra,
characteristic of protein β-sheet secondary structure was recorded with increasing temperature from 20 °C to 80 °C. Wild-type
αB-crystallin and Δ10 show similar smooth change in molar ellipticity without abrupt change in protein secondary structure.
The mutant protein (Δ11) shows a conformational transition around 65 °C, whereas Δ12 shows such a transition around 57 °C
indicative of its susceptibility to unfolding at a lower temperature.
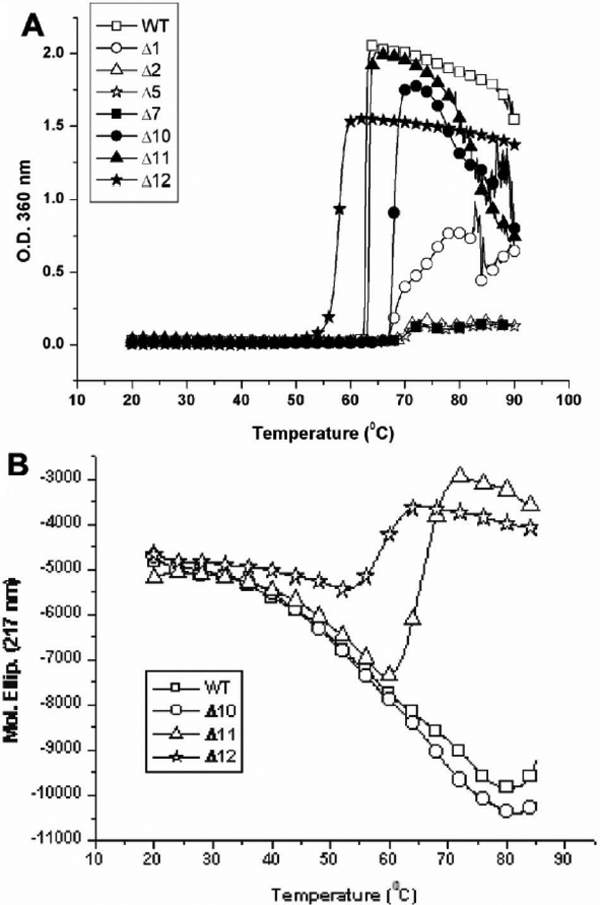
 Figure 6 of
Liao, Mol Vis 2009; 15:1429-1444.
Figure 6 of
Liao, Mol Vis 2009; 15:1429-1444.  Figure 6 of
Liao, Mol Vis 2009; 15:1429-1444.
Figure 6 of
Liao, Mol Vis 2009; 15:1429-1444. 