Figure 5. Comparison of chaperone activities of wild-type αB-crystallin and its truncated mutants under chemical and thermal denaturation.
A: Inhibition of DTT-induced insulin B chain aggregation by wild-type αB-crystallin and its truncated mutants. The chaperone
activities of Δ10 and wild-type αB-crystallin against chemical denaturation of insulin at 38 °C were similar in a molar ratio
of 1:11 (chaperone/insulin). The mutant protein (Δ11) shows poor chaperone activity and Δ12 shows almost no protective activity
under identical conditions. The final concentration of bovine pancreas insulin after mixing is 52.4 μM. B: Inhibition of thermal denaturation of porcine βL-crystallin by wild-type αB-crystallin and its truncated mutants. The mutant
protein (Δ10) shows the best chaperone activity among four proteins tested at 59 °C in a molar ratio of 2:7 (chaperone/βL-crystallin).
Wild-type αB-crystallin and Δ11 show poor chaperone activity under identical conditions, whereas Δ12 shows no protective activity.
The final concentration of porcine βL-crystallin is 4.3 μM.
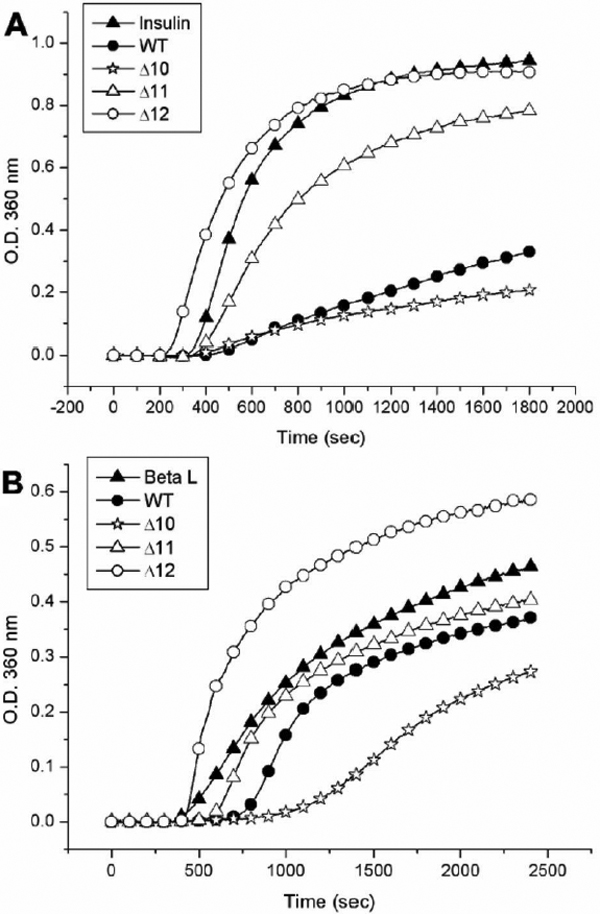
 Figure 5 of
Liao, Mol Vis 2009; 15:1429-1444.
Figure 5 of
Liao, Mol Vis 2009; 15:1429-1444.  Figure 5 of
Liao, Mol Vis 2009; 15:1429-1444.
Figure 5 of
Liao, Mol Vis 2009; 15:1429-1444. 