Figure 4. Comparison of chaperone activities of various αB-crystallin and deletion mutants against chemical and thermal denaturation.
A: Inhibition of DTT-induced insulin B chain aggregation by wild-type αB-crystallin and its truncated mutants. The mutant proteins
(Δ2, Δ5, Δ7, and Δ10) all show similar chaperone activities with wild-type αB-crystallin at 37 °C in a molar ratio (chaperone/insulin)
of 1:16. The final concentration of bovine pancreas insulin is 59.3 μM. B: Inhibition of thermal denaturation of porcine βL-crystallin by wild-type αB-crystallin and its truncated mutants. βL-crystallin
was used as a substrate for chaperone-activity assays at 60 °C. Mutant proteins (Δ2, Δ5, Δ7, and Δ10) all show higher chaperone
activity than wild-type αB-crystallin in a molar ratio of (chaperone/βL-crystallin) of 2:7, with Δ2 showing highest activity
among 4 deletion mutants. The final concentration of porcine βL-crystallin is 6.2 μM. C: Chaperone-activity assays using porcine γ-crystallin as a substrate. The wild-type αB-crystallin and its truncated mutants
were heated at 65 °C in a molar ratio of 1:1 (chaperone/γ-crystallin). The mutant proteins (Δ2, Δ5, Δ7, and Δ10) all show
better chaperone activities than wild-type αB-crystallin which exhibits no chaperone activity for heat denaturation of γ-crystallin.
The final concentration of porcine γ-crystallin is 2.6 μM.
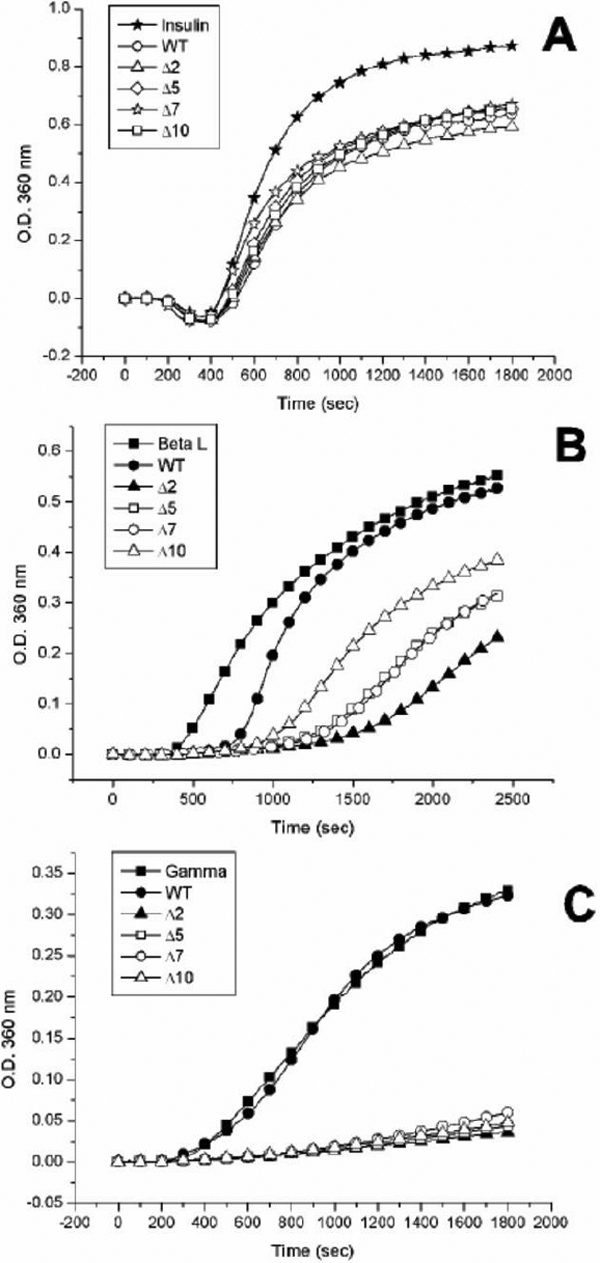
 Figure 4 of
Liao, Mol Vis 2009; 15:1429-1444.
Figure 4 of
Liao, Mol Vis 2009; 15:1429-1444.  Figure 4 of
Liao, Mol Vis 2009; 15:1429-1444.
Figure 4 of
Liao, Mol Vis 2009; 15:1429-1444. 