Figure 2. Chaperone-like activity of
αB-crystallin-βA3/A1102–117 peptide complex against
denaturing βL-crystallin. βL-crystallin (100 μg)
was incubated at 55 °C in presence of 5 μg and 10 μg of αB-crystallin
or αB-crystallin-βA3/A1102–117 peptide complex for 60 min
and the light scattering was measured as described under
methods. The results show that prior interaction of
αB-crystallin with βA3/A1102-117 peptide diminished its
chaperone-like activity against denaturing βL-crystallin. A,
βL-crystallin; B, βL-crystallin + 5 μg
αB-crystallin; C, βL-crystallin + 10 μg
αB-crystallin; D, βL-crystallin + 5 μg
αB-crystallin-βA3/A1102–117 peptide complex; E, βL-crystallin
+ 10 μg αB-crystallin-βA3/A1102–117 peptide complex; F,
αB-crystallin or αB-crystallin-βA3/A1102–117 peptide complex
alone. Insert: Relative light scattering by βL-crystallin in
presence of αB-crystallin or αB-crystallin-βA3/A1102–117
peptide complex. Scattering by βL-crystallin alone at 60 min
is considered to be 100%.
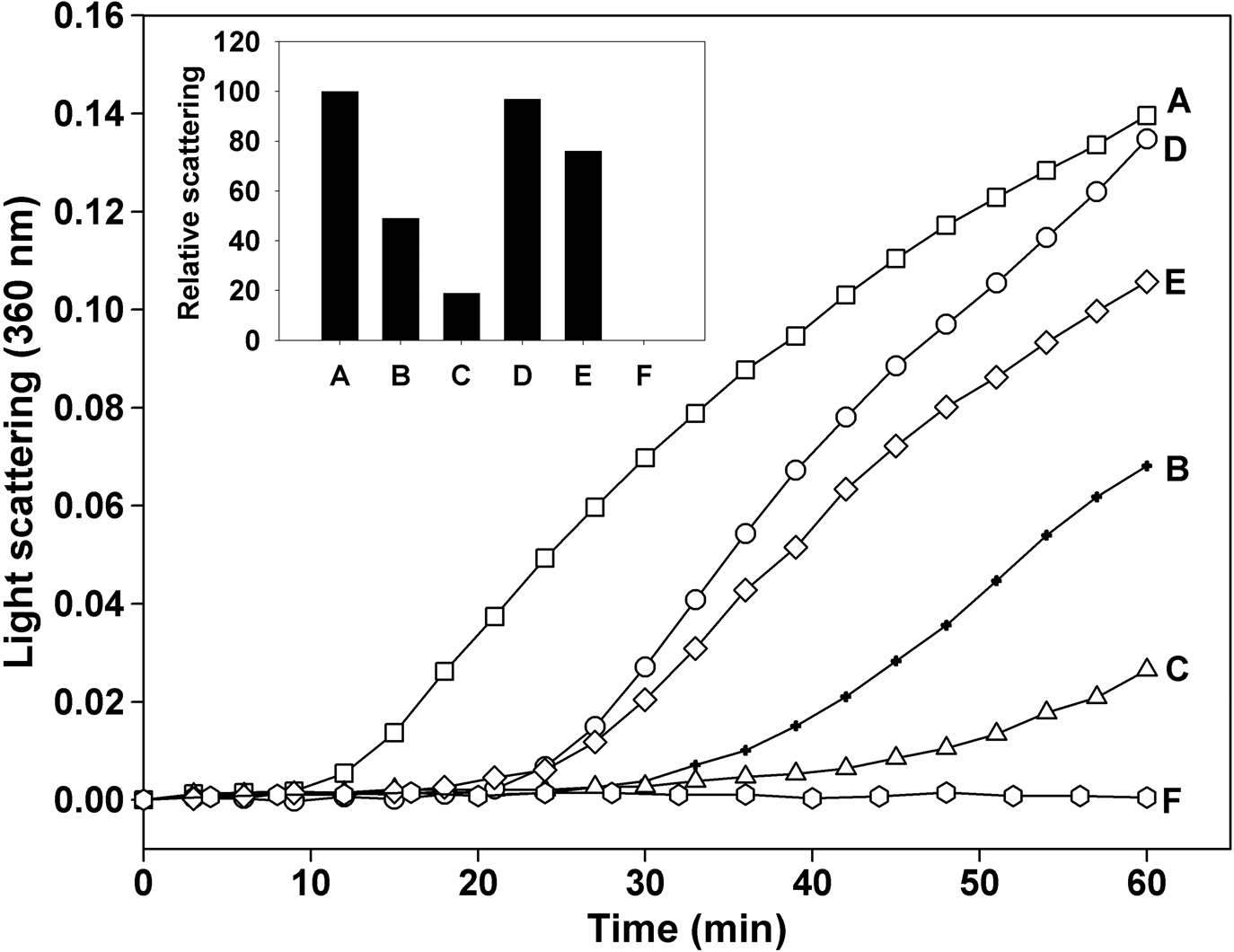
 Figure 2 of Rao, Mol Vis 2008; 14:666-674.
Figure 2 of Rao, Mol Vis 2008; 14:666-674.  Figure 2 of Rao, Mol Vis 2008; 14:666-674.
Figure 2 of Rao, Mol Vis 2008; 14:666-674. 