Figure 2. Olomoucine treatment does not
disrupt the integrity of the epithelial cell sheet. A: The
immunofluorescence of E-cadherin in epithelial cells of corneal whole
mounts of control animals shows tightly packed epithelial cells with a
smooth, advancing cell front. Cells were compact and cell density was
high especially along the wound edge. B: Higher magnification
of the boxed area shown in panel A shows that E-cadherin
immunofluorescence in control corneas was confined almost entirely to
cell-to-cell boundaries. Punctate E-cadherin staining was seen at the
migrating front on the edge lacking cell-to-cell contacts (arrowhead). C:
Superimposition of E-cadherin immunostaining and DAPI-staining of
nuclei confirms that E-cadherin is located at cell-to-cell boundaries
and that all cells express E-cadherin. D: In olomoucine-treated
corneas, cell density was appreciably lower and the migrating front was
irregular. Cell-to-cell junctions appeared to be intact except for a
few cells at the wound edge. Immunostaining of E-cadherin was weak or
undetectable at the migrating front on the edge lacking cell-to-cell
contacts (arrowhead). E: Higher magnification of the boxed area
shown in panel D demonstrates punctate intracellular
immunostaining for E-cadherin in many cells (single arrows). E-cadherin
localization at cell-to-cell junctions was disrupted in a few cells
along the wound edge (double arrows). F: Superimposition of
E-cadherin immunofluorescence and DAPI-staining of nuclei confirms that
E-cadherin is located at cell-to-cell boundaries and demonstrates that
all cells express E-cadherin. Scale bar=100 μM in A,C,D,
F; 40 μM in B,E.
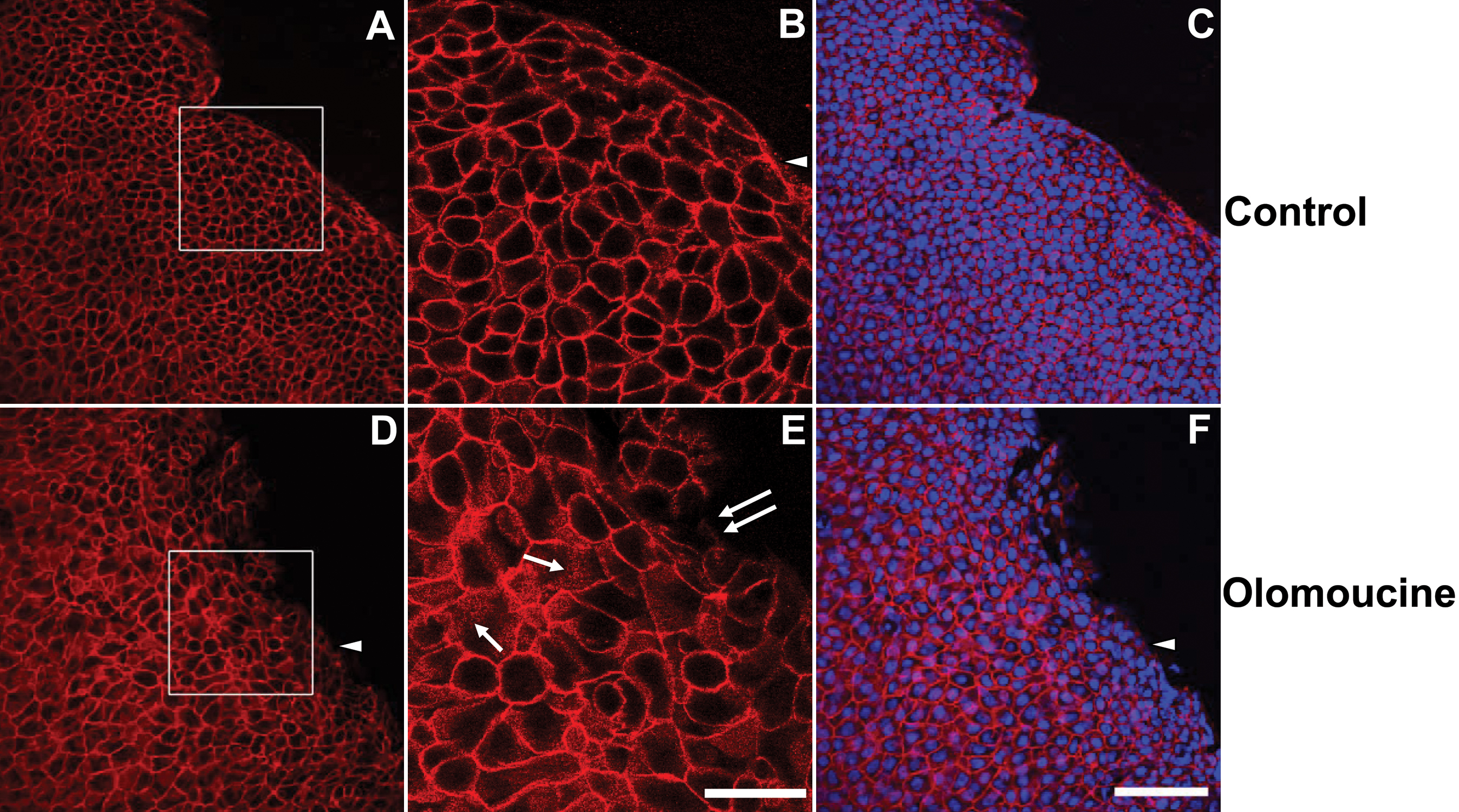
 Figure 2 of Tripathi, Mol Vis 2008; 14:542-549.
Figure 2 of Tripathi, Mol Vis 2008; 14:542-549.  Figure 2 of Tripathi, Mol Vis 2008; 14:542-549.
Figure 2 of Tripathi, Mol Vis 2008; 14:542-549. 