![]() Figure 3 of
Mozaffarieh, Mol Vis 2008;
14:224-233.
Figure 3 of
Mozaffarieh, Mol Vis 2008;
14:224-233.
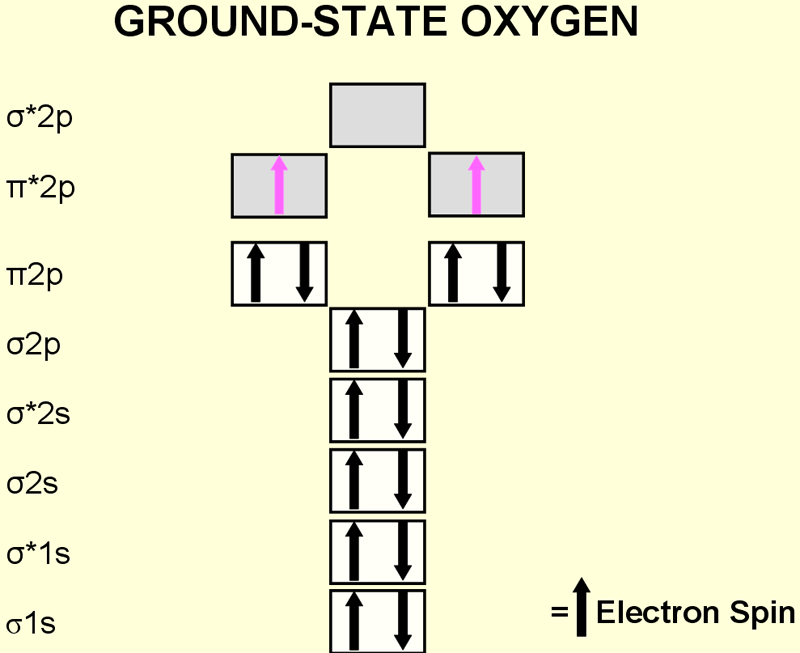
Figure 3. Electron configuration of the diatomic oxygen molecule
Atomic oxygen (atomic number 8) has a total of eight electrons. The oxygen molecule (O2) has 16 electrons. In ground state (the most stable state of oxygen), the last two electrons of the oxygen molecule are located in a different π* antibonding orbital. These two unpaired electrons have the same quantum spin number (they have parallel spins) and qualify ground state oxygen to be a di radical.