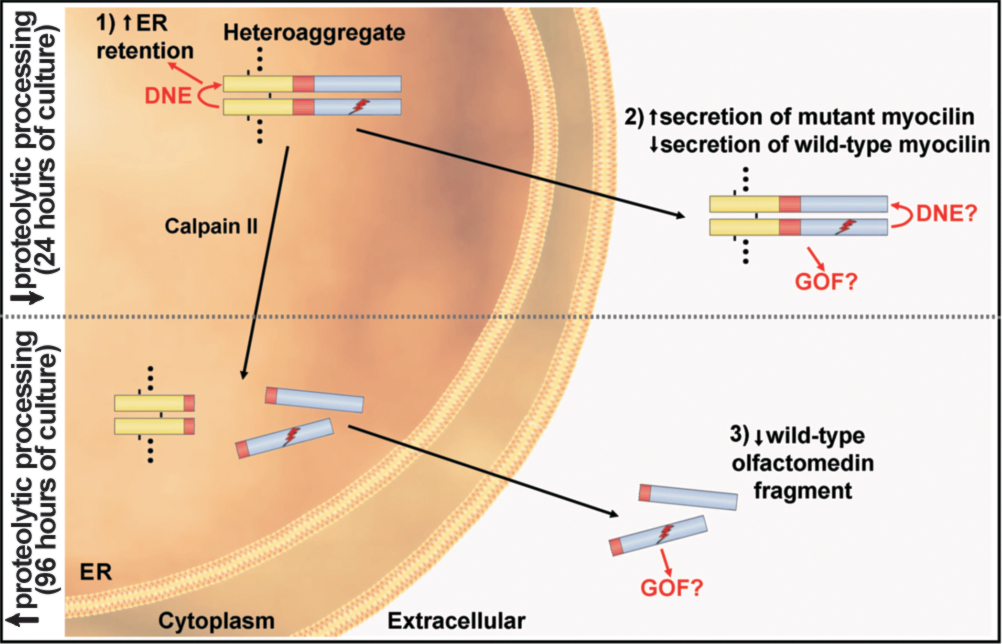
Figure 6. Model of the secretion and
proteolytic processing of wild-type/mutant myocilin heteroaggregates.
Heteroaggregation takes place in the lumen of the ER. For simplicity
the diagram shows a heterodimer but the aggregates are composed of
multiple myocilin monomers (indicated by dots in the heteroaggregate)
linked by disulphide bonds (short black lines in the heterodimer).
According to this model heteroaggregation has three major effects: 1)
increases the retention of wild-type myocilin in the ER via a dominant
negative effect (DNE); 2) increases secretion of mutant myocilin along
with a reduction of extracellular wild-type myocilin, and 3) reduces
the amount of extracellular wild-type myocilin (olfactomedin fragment)
under conditions known to increase the proteolytic cleavage of myocilin
by calpain II such as 96 h of culture [
15].
The N-terminal fragment that arises after
the cleavage mainly remains in the ER [
15].
A possible gain of function (GOF) of
mutant myocilin (both full-length and cleaved olfactomedin fragment)
that could contribute to glaucoma pathogenesis is also indicated. The
leucine zipper (yellow), linker (red), and olfactomedin (blue) domains
of myocilin are also indicated in the heterodimer. The ray indicates
mutant myocilin. Question marks indicate a hypothetical dominant
negative effect and/or gain of function of the mutant protein.
 Figure 6 of Aroca-Aguilar, Mol Vis 2008; 14:2097-2108.
Figure 6 of Aroca-Aguilar, Mol Vis 2008; 14:2097-2108.  Figure 6 of Aroca-Aguilar, Mol Vis 2008; 14:2097-2108.
Figure 6 of Aroca-Aguilar, Mol Vis 2008; 14:2097-2108.