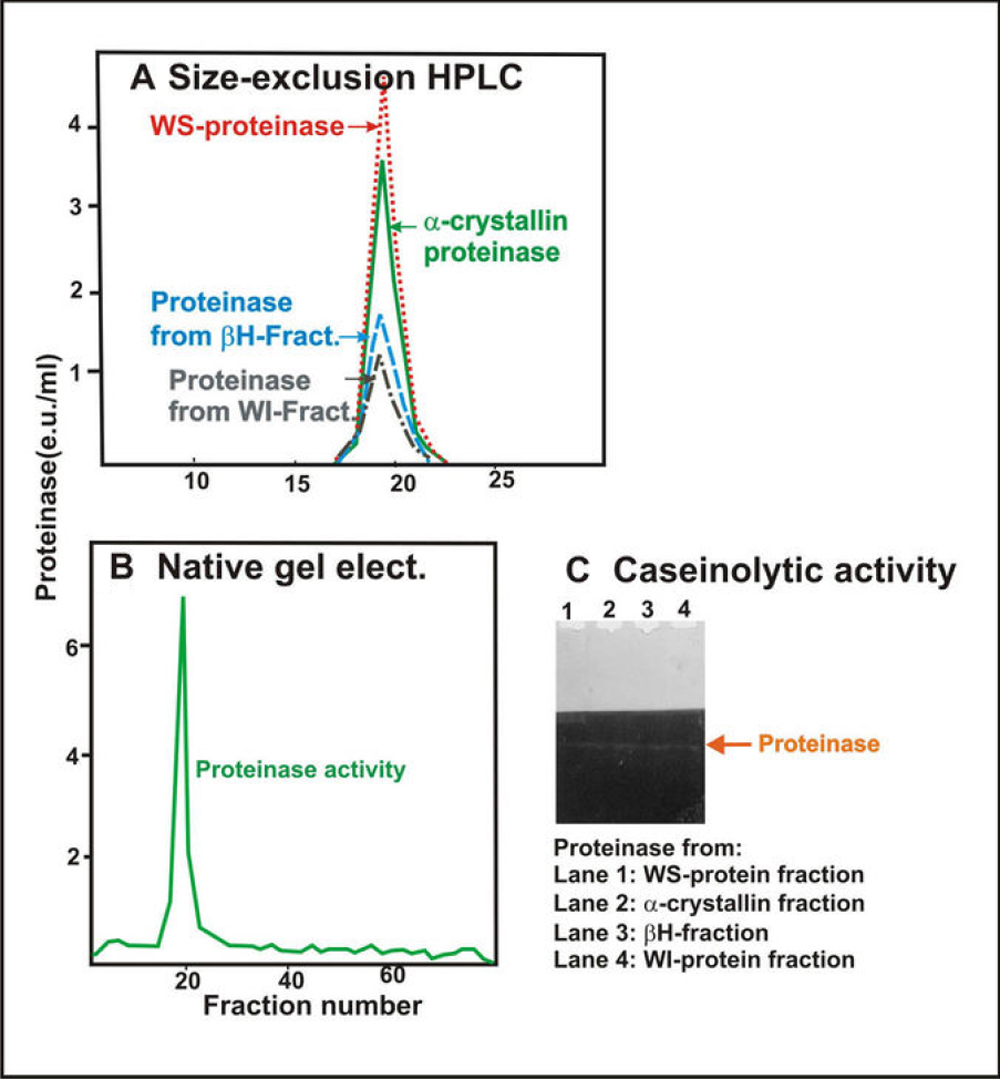
Figure 9. Comparison of properties of
Arg-bond hydrolyzing proteinase isolated from the WS protein fraction,
the α-crystallin fraction, the βH-crystallin fraction, and
the membrane fraction of human lenses. A: Size-exclusion HPLC
analysis is shown of the Arg-bond hydrolyzing proteinase purified from
the WS protein fraction (red dotted line), the α-crystallin fraction
(green dotted line), the βH-crystallin fraction (blue dotted
line), and the membrane fraction (black dotted line). B:
Nondenaturing gel electrophoresis is shown of the combined proteinase
preparations from the WS protein fraction, α-crystallin fraction, βH-crystallin
fraction, and membrane fraction. Note that a single proteinase peak was
observed on combining proteinases isolated from all four fractions. C:
Caseinolytic activity of the four proteinases isolated from WS-protein
fraction (lane 1), α-crystallin fraction (lane 2), βH-crystallin
fraction (lane 3), and membrane fraction (lane 4). Note that a
co-migration caseinolytic activity of the four proteinases on a
non-denaturation gel was observed.
![]() Figure 9 of Srivastava, Mol Vis 2008;
14:1872-1885.
Figure 9 of Srivastava, Mol Vis 2008;
14:1872-1885. 