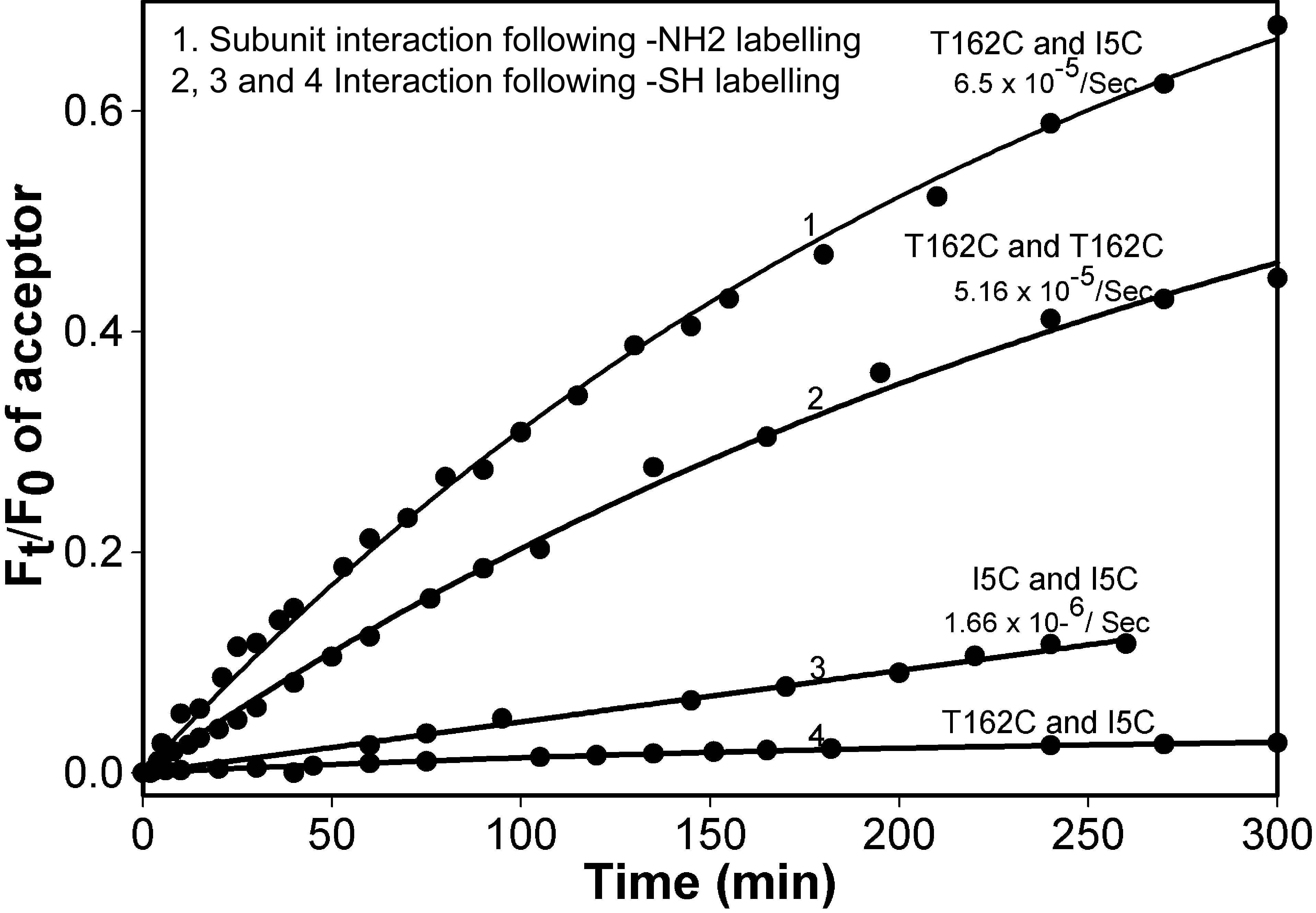
Figure 9. Subunit exchange studies with
αB-crysteine mutants. FRET assay was performed with 50 μg of each
donor and acceptor Alexa Fluor labeled protein at 37 °C.
The rate of subunit exchange was calculated by measuring the increase
of acceptor fluorescence intensity. Ft/F0 represents the ratio of
acceptor fluorescence intensity at given time intervals to the
fluorescence intensity at the baseline. Curve 1 is the NH2-labeled
protein; Curves 2, 3, and 4 are thiol-labeled proteins. The rate
of subunit exchange between NH2 -labeled αBT162C–αBI5C
was 65.5x10-5sec-1. The interaction
rate between thiol-labeled αBT162C subunits was 5.16x10-5sec-1.
The rate of interaction between thiol-labeled αBI5C
subunits was 1.66x10-6sec-1. There was no
measurable interaction between αBT162C and αBI5C when one of
the mutants was labeled with a donor Alexa Fluor and the
second mutant was labeled with an acceptor Alexa Flour at thiol
residues. The results demonstrate the absence of interaction between NH2-
and COOH-terminal regions of the two subunits and the presence of high
degree of interactions between two NH2-terminal regions or
two COOH-terminal regions of αB-crystallin in an oligomer.
![]() Figure 9 of Murugesan,
Mol Vis 2008; 14:1835-1844.
Figure 9 of Murugesan,
Mol Vis 2008; 14:1835-1844. 