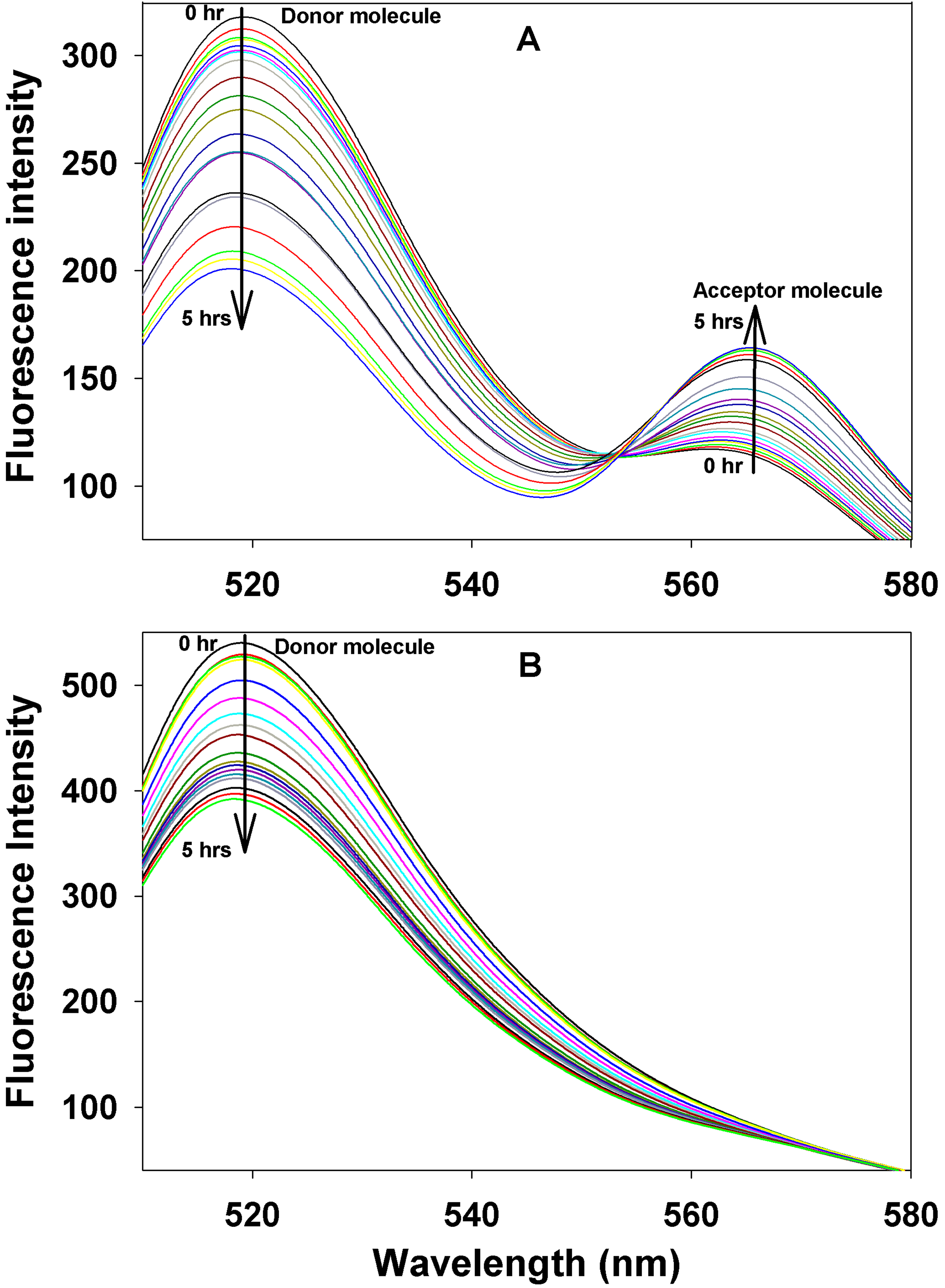
Figure 8. Subunit interaction determined
by FRET assay. A: Time-dependent changes in the emission
spectra of donor and acceptor Alexa Fluor dyes (thiol) that were
labeled in αBT162C due to FRET are shown. The emission spectra of
αBT162C, excited at 485 nm, were recorded at 5–20 min intervals after
mixing an equal amount of donor and acceptor protein at 37 °C. The
arrow marks show that donor fluorescence intensity decreases at 519 nm
and acceptor emission increases at 565 nm. Emission spectra were
recorded for 5 h with the excitation wavelength of 495 nm. B:
There was a time-dependent decrease in the emission spectra of
thiol-reactive, Alexa Fluor-labeled αBI5C in the presence of Alexa
Fluor-labeled αBT162C. Note the absence of Alexa Fluor spectra with 565
nm maximum indicating
that there is no energy transfer to the
acceptor Alexa Fluor 555 from the donor Alexa Fluor 488. The results
suggest that there was no interaction between cysteines in NH2-
and COOH-terminal regions of the αB-crystallin subunits.
![]() Figure 8 of Murugesan,
Mol Vis 2008; 14:1835-1844.
Figure 8 of Murugesan,
Mol Vis 2008; 14:1835-1844. 