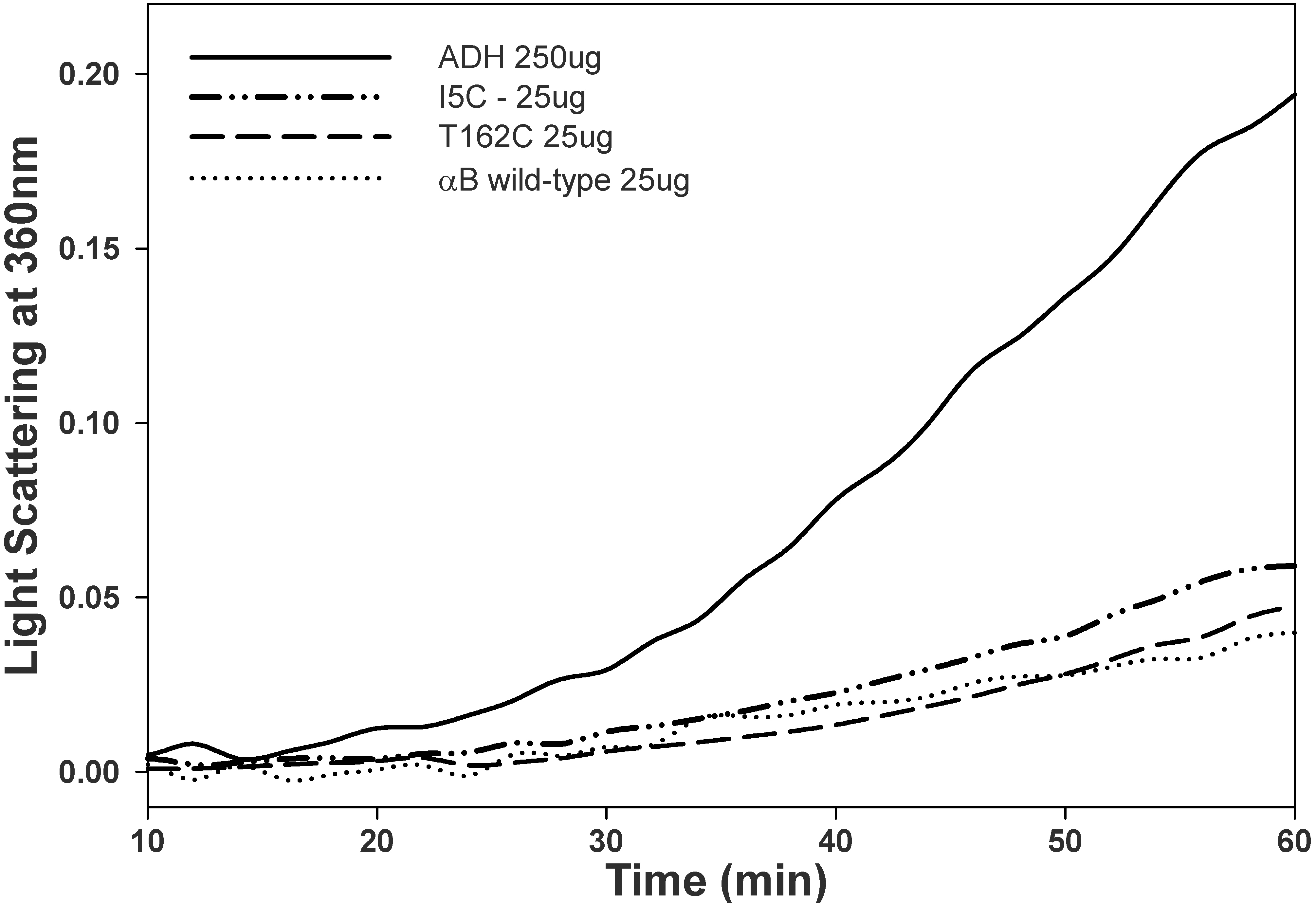
Figure 7. Chaperone activity of αBI5C and
αBT162C proteins and wild-type αB-crystallin. EDTA-induced aggregation
of ADH in the absence or presence of wild-type or mutant proteins was
measured at 37 °C. In these experiments, 250 µg of ADH was used
with or without crystallins. The chaperone activities of the mutants
and wild-type proteins were similar when ADH was used as client
protein. This study indicates that the mutation did not alter the
structure-function of the protein maintained under reducing
condition.
![]() Figure 7 of Murugesan,
Mol Vis 2008; 14:1835-1844.
Figure 7 of Murugesan,
Mol Vis 2008; 14:1835-1844. 