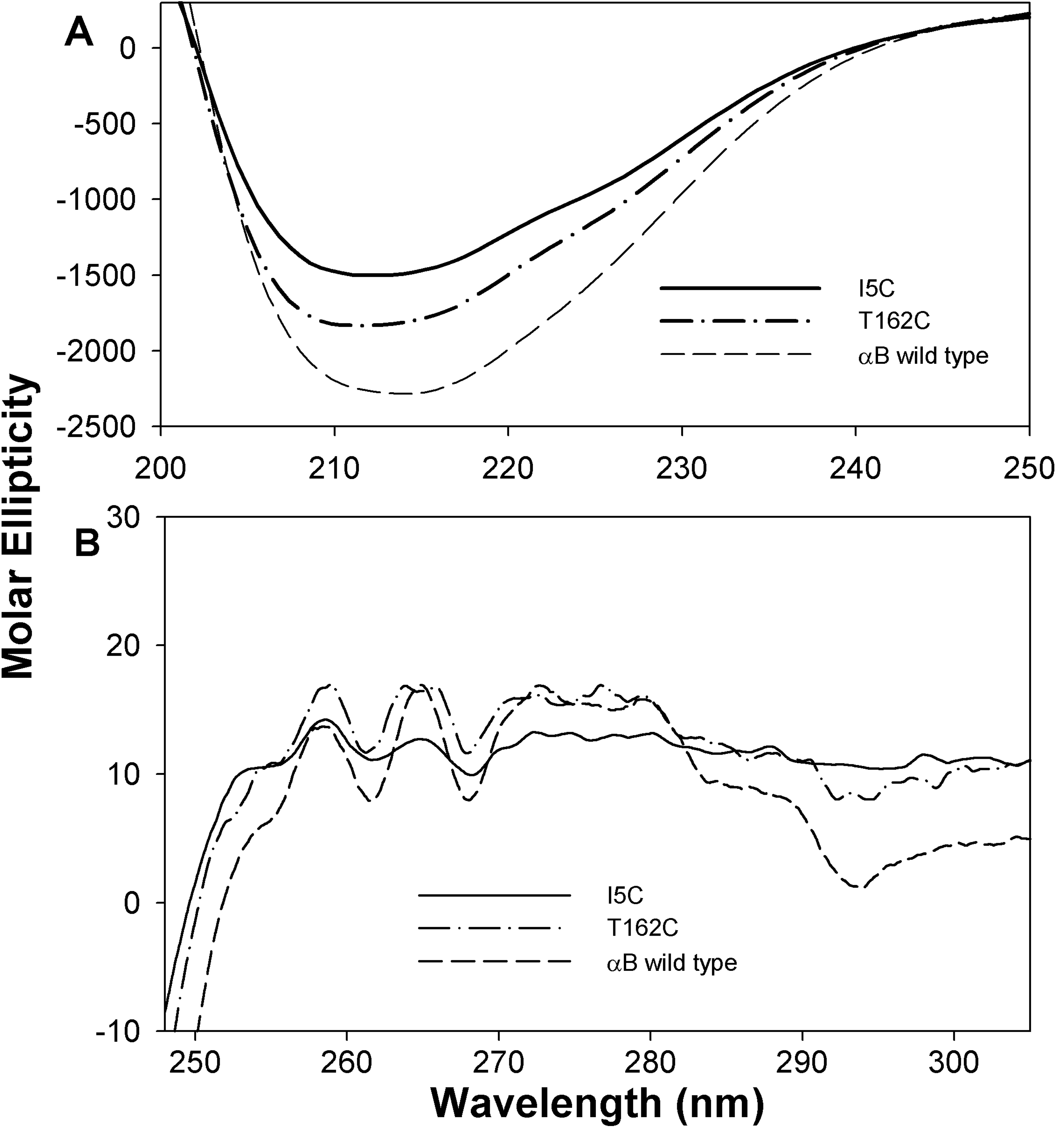
Figure 6. Circular dichroism spectra of
wild-type and mutant I5C and T162C crystallins. A: Far-UV CD
spectra were recorded using 0.2 mg/ml protein in a 0.5 cm cell path
length at 25 °C. B: Near-UV CD spectra of wild type and
mutant were recorded using a protein sample of 3 mg/ml in 0.5 cm cell
path length at 25 °C. Both far- and near-UV CD spectra
of αBI5C and αBT162C show negligible differences in secondary and
tertiary structures. The spectra under reducing conditions are similar
to that of wild-type αB-crystallin. These results further confirm
the minimal impact of αBI5C and αBT162C mutations on the structure of
αB-crystallin.
![]() Figure 6 of Murugesan,
Mol Vis 2008; 14:1835-1844.
Figure 6 of Murugesan,
Mol Vis 2008; 14:1835-1844. 