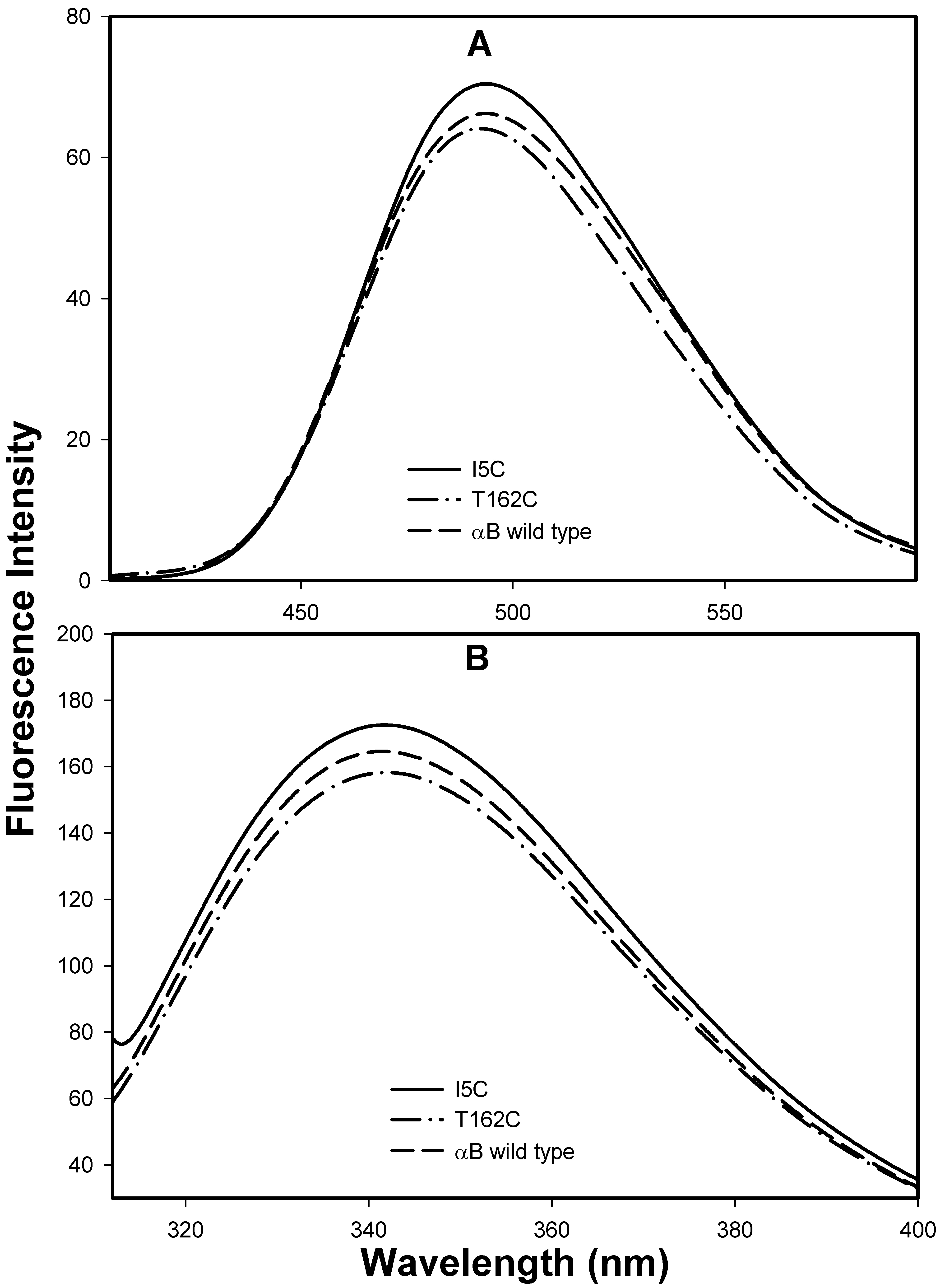
Figure 5. Bis-ANS binding and
intrinsic tryptophan fluorescence profile of mutant αBI5C and
αBT162C proteins. A: Bis-ANS 10 μl (1 mM) was added to protein
samples of 0.2 mg/ml in 50 mM phosphate buffer (pH.7.2) were excited at
385 nm, and emission was scanned between 400 nm and 600 nm. The mutant
αBI5C and αBT162C proteins show similar bis-ANS intensity like
wild-type αB-crystallin. B: The intrinsic tryptophan
fluorescence spectra of wild-type αB-crystallin, αBI5C and
αBT162C. Protein samples (0.2 mg/ml) in 50 mM phosphate buffer (pH 7.2
) were used. The samples were excited at 295 nm and the
emission was scanned between 310 nm and 400 nm. Tryptophan
spectrum of mutant αBI5C and αBT162C proteins were comparable
to the wild-type αB-crystallin spectrum. The
data suggests that the mutation did not alter the
native conformations of mutant proteins under reducing
conditions.
![]() Figure 5 of Murugesan,
Mol Vis 2008; 14:1835-1844.
Figure 5 of Murugesan,
Mol Vis 2008; 14:1835-1844. 