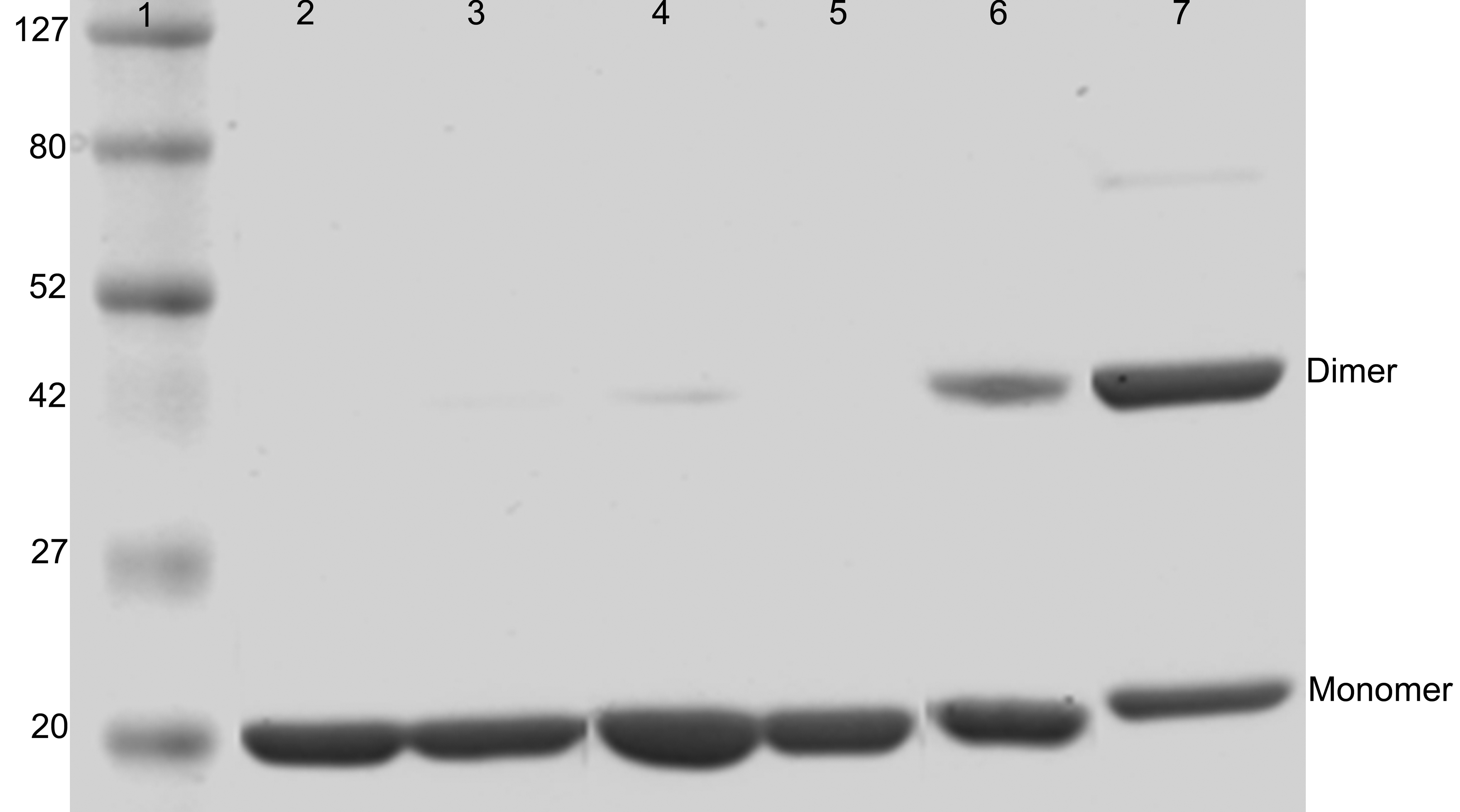
Figure 1. SDS-PAGE of αBI5C and αBT162C
mutant and wild-type αB-crystallins under different conditions. Lane 1
protein markers in kDa; lanes 2 and 5 show the wild-type αB-crystallin;
lanes 3 and 6 show αBI5C; lanes 4 and 7 show αBT162C; lanes 2, 3, and 4
show proteins under reducing conditions; and lanes 5, 6, and 7 show
proteins under non-reducing conditions. Both mutants form dimers
under non-reducing conditions and the higher intensity of αBT162C dimer
band than that of αBI5C dimer suggests that the COOH-terminal
extensions in the subunits interact more compared to the
interactions between NH2-terminal regions.
![]() Figure 1 of Murugesan,
Mol Vis 2008; 14:1835-1844.
Figure 1 of Murugesan,
Mol Vis 2008; 14:1835-1844. 