![]() Figure 2 of
Geller, Mol Vis 2007;
13:730-739.
Figure 2 of
Geller, Mol Vis 2007;
13:730-739.
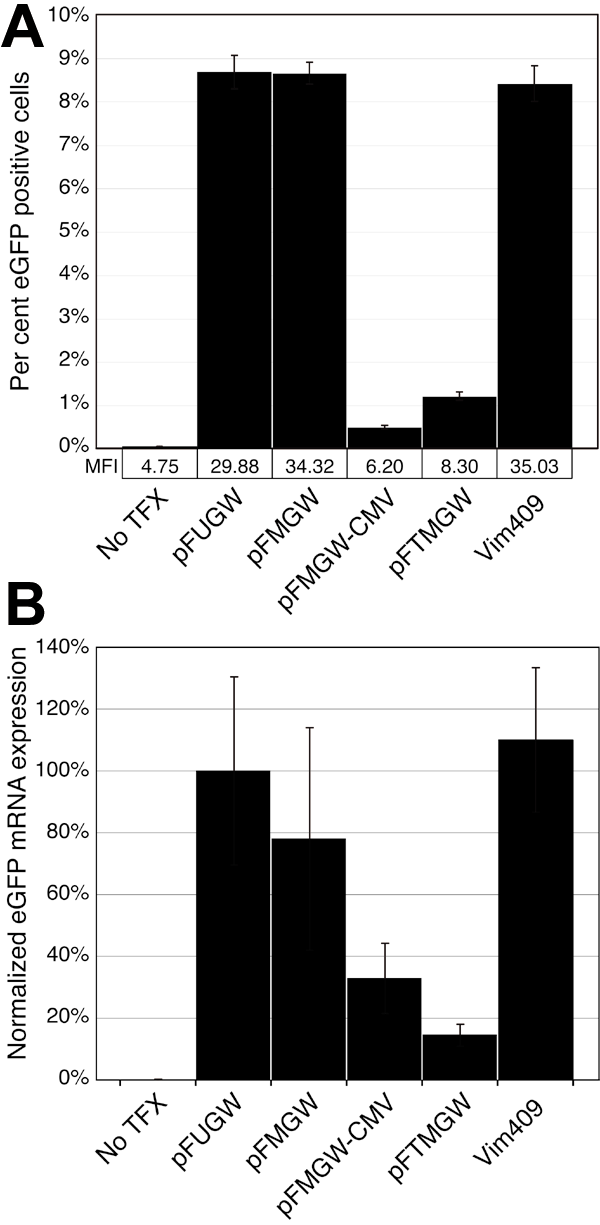
Figure 2. Quantitative analysis of eGFP expression in transfected cells
A: Flow cytometry analysis of eGFP expressing Müller cells. Nearly confluent cells in 6-well plates were un-transfected (No TFX), transfected with 2 μg of control vectors (pFUGW, pFMGW, pFMGW-CMV, pFTMGW), or transfected with 2 μg of a 409 bp fragment of the rat vimentin promoter cloned into pFTMGW (Vim409). Greater than 105 cells were analyzed in each of six replicate samples for each condition. Gating was set such that positive cell counts in the No TFX samples were less than 0.07% of all cells. B: Quantitative RT-PCR analysis of eGFP expression. Total RNA was isolated from cultured Müller cells transfected with the same set of plasmids as in A, and reverse transcribed. eGFP and β-actin transcript expression were measured using Taqman fluorescent probes. Threshold cycle numbers were first internally normalized (within each individual reaction) to β-actin, and secondarily normalized to pFUGW eGFP expression levels (set to 100%). Samples were analyzed in triplicate. pFUGW is the parent plasmid. pFMGW is pFUGW with the MCS in place of the ubiquitin-C promoter. pFMGW-CMV is the pFMGW plasmid lacking the CMV promoter. pFTMGW is pFMGW with the TB cloned immediately upstream of the MCS. Vim409 is the 409 bp fragment of the rat vimentin promoter/5' UTR cloned into pFTMGW. Error bars represent 1 SD for panels A and B.