![]() Figure 6 of
Yuan, Mol Vis 2007;
13:2083-2095.
Figure 6 of
Yuan, Mol Vis 2007;
13:2083-2095.
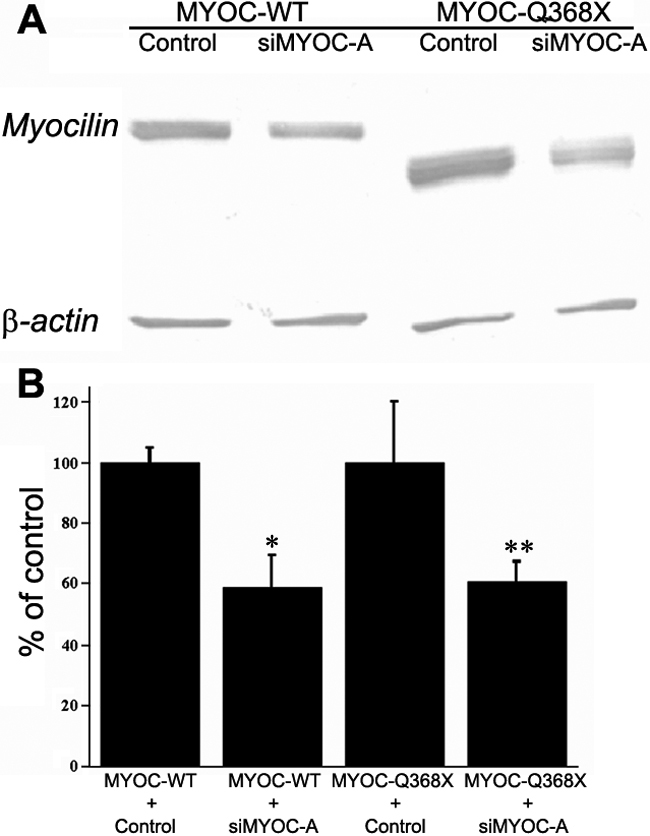
Figure 6. Suppression of wild-type (MYOC-WT) and mutant Q368X (MYOC-Q368X) myocilin proteins by shMYOC-A (siMYOC-A) in TM5 cells
A: Western blot of protein lysates from cultured HEK293 cells after cotransfections of MYOCpEGFP or MYOCQ368XpEGFP with control pH1-RNA or shMYOC-A plasmids is shown. The myocilin fusion protein and β-actin were detected with anti-EGFP and anti β-actin antibodies, respectively. After cotransfection of MYOCpEGFP with control pH1-RNA, abundant wild-type MYOC-EGFP was noted in HEK 293 cell lysates. Moderate suppression of wild-type MYOC-EGFP by shMYOC-A was noted after cotransfection of MYOCpEGFP with shMYOC-A (MYOC-WT). Similarly, abundant mutant Q368X-EGFP was noted in HEK 293 cell lysates after cotransfection of MYOCQ368XpEGFP with control pH1-RNA. Moderate reduction of mutant Q368X-EGFP was noted after cotransfection of MYOCQ368XpEGFP with shMYOC-A (MYOC-Q368X). B: Suppression efficiency of wild-type and mutant myocilins by shMYOC-A is shown in a graph. The protein bands from A were digitized to quantify the suppression efficiency of shMYOC-A on wild-type MYOC-EGFP and mutant Q368X-EGFP using UN-SCAN-IT software. The pixel intensities from the myocilin fusion proteins were normalized to the pixel intensities from the β-actin bands. The ratio of intensities between the control shRNA and shMYOC-A was used to determine the suppression efficiency. Compared with their respective control of wild-type (MYOC-WT) and mutant Q368X (MYOC-Q368X) myocilins, shMYOC-A reduced the expression of MYOC-WT and MYOC-Q368X to 58.9%±10.6% (the asterisk indicates a p<0.02) and 60.8%±6.4% (the double asterisk indicates a p<0.03), respectively (n=3, bars=SD).