![]() Figure 2 of
Spellicy, Mol Vis 2007;
13:1866-1872.
Figure 2 of
Spellicy, Mol Vis 2007;
13:1866-1872.
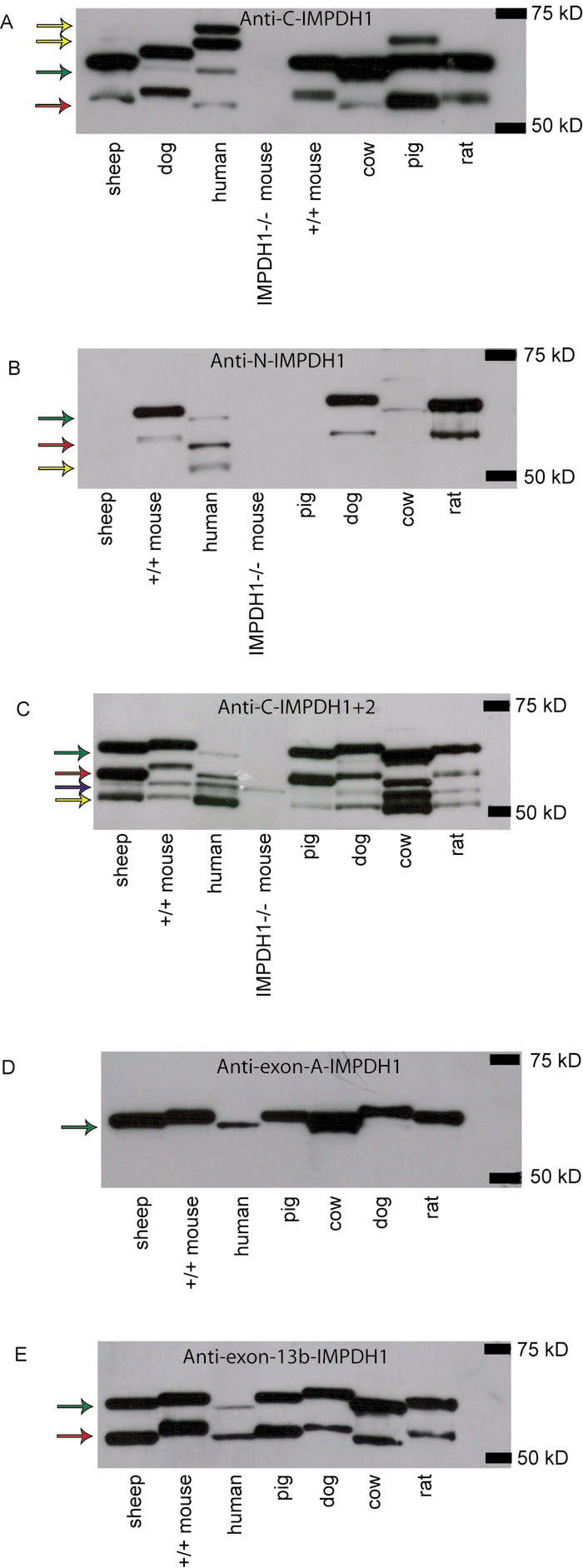
Figure 2. Western blots using various anti-IMPDH antibodies
Arrows indicate bands of importance. Green arrows indicate the human 65 kDa IMPDH1 retinal isoform (IMPDH1+A+13b), red arrows indicate human 56 kDa retinal isoform (IMPDH1+13b), the blue arrow indicates IMPDH2, and yellow arrows indicate bands of unknown origin. A: Western blot using the anti-C-IMPDH1 antibody. Arrows indicate the 65 kDa (green) and 55 kDa (red) human retinal isoforms of IMPDH1. The two highest molecular weight bands detected in human retinal lysate and the one highest molecular weight band detected in pig retinal lysate are thought to be nonspecific binding (yellow) of the antibody given that they are not observed in any of the other blots using IMPDH1-specific antibodies. B: Western blot using the anti-N-IMPDH1 antibody. The two bands present in sheep, dog, human, mouse, and rat correspond in molecular weight to the human IMPDH1 retinal isoforms previously described, 65 kDa and 56 kDa, indicated by a green and red arrow, respectively [18]. This antibody does not appear to accurately identify the IMPDH1 proteins in sheep, cow, or pig. Cow has an amino acid at one residue that differs from the epitope used to create the antibody, probably causing the non-specificity of binding. We suspect the same is true in pig although the sequence is not available to show this. The failure of this antibody to recognize IMPDH1 in sheep is a mystery given complete conservation of the epitope. Lastly there is a band around 52 kDa in human retina that we believe is nonspecific binding of the antibody (indicated by the yellow arrow). C: Western blot using the anti-C-IMPDH1+2 antibody. From higher molecular weight to lower molecular weight the arrows represent human IMPDH1 including exons A and 13b (65 kDa retinal isoform, green), IMPDH1 including exon 13b only (56 kDa retinal isoform, red), and IMPDH2 (55.5 kDa, blue arrow), respectively. The lowest molecular weight band observed in all species but knock out mouse is thought to be nonspecific binding of the antibody (yellow). It is possible that this band may be canonical IMPDH, or a form of IMPDH1 altered by post-translational modification, given that it is not observed in the IMPDH1 knockout mouse, but this is unlikely given that neither the anti-C-IMPDH1 nor the anti-N-IMPDH1 specific antibodies detect this band. D: Western blot using anti-exon-A-IMPDH1 specific antibody. As expected one band is observed in this blot corresponding to the 65 kDa human retinal IMPDH1 isoform previously described. In human, this isoform is lower in abundance as described previously [18]. E: Western blot using the anti-exon-13b-IMPDH1 antibody. All species tested show two IMPDH1 isoforms, presumably corresponding to the human IMPDH1+A+13b and IMPDH1+13b isoforms. Again, and in contrast to the other species tested, the 56 kDa retinal IMPDH1 isoform in human appears to be more abundant than the 65 kDa IMPDH1 retinal isoform, confirming previous observations [18].