![]() Figure 6 of
Redmond, Mol Vis 2007;
13:1813-1821.
Figure 6 of
Redmond, Mol Vis 2007;
13:1813-1821.
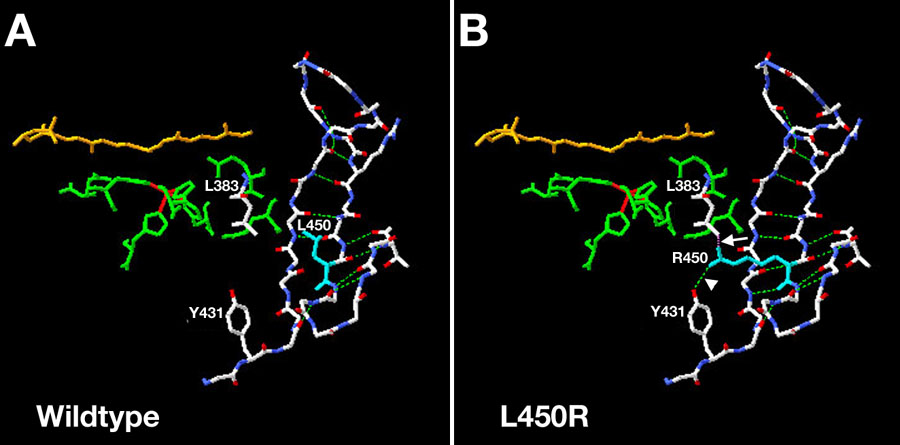
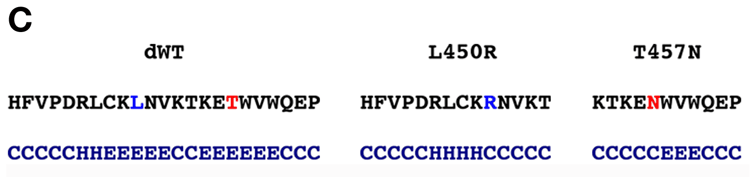
Figure 6. Effect of human pathogenic mutation L450R at aa450 on predicted tertiary structure of RPE65, modeled on ACO, and on secondary structure prediction of RPE65
A and B: Dog RPE65 residues are plotted onto the ACO backbone [26] using DeepView. A: L450 (wildtype) showing lack of interaction of L450 with other residues; B: R450 showing interaction of R450 with L383 and Y431. Arrow indicates predicted steric hindrance of R450 with L383 and arrowhead indicate extra side-chain H-bonding with Y431. C: Effect of L450R and T457N mutations on predicted secondary structure of RPE65. R450 abolishes the predicted β-strand number 26 in both dog and mouse RPE65 converting it to predicted α-helix, while N457 shortens but does not eliminate the predicted extended β-sheet E indicates predicted extended β-strand, H indicates α-helix, and C indicates other structure.