![]() Figure 2 of
Zhou, Mol Vis 2007;
13:1298-1310.
Figure 2 of
Zhou, Mol Vis 2007;
13:1298-1310.
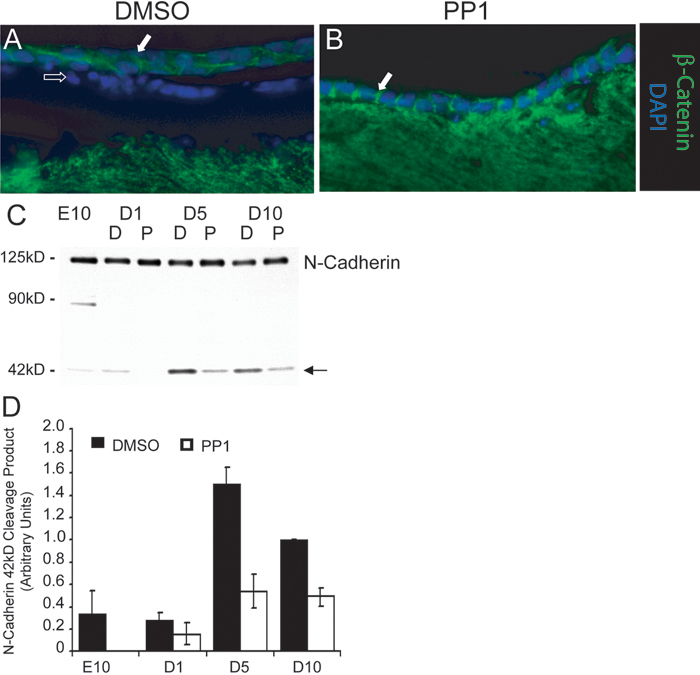
Figure 2. Loss of cadherin junctions in cataract culture is related to N-cadherin cleavage
Lenses were grown in cataract culture conditions in the presence (B) and absence (A) of the Src kinase inhibitor PP1. Lens sections were observed by fluorescence microscopy following immunostaining with an antibody to the cadherin complex protein β-catenin (green) and by the labeling of nuclei with DAPI (blue). Solid arrow denotes lateral cell-cell borders. In the cataractous lenses, β-catenin was either absent or greatly diminished at lateral cell-cell interfaces, the location of cadherin junctions in normal epithelial cells. Occasionally, staining was retained, but most often this was found atypically distributed as between apical and basal interfaces of multilayered epithelial cells. No β-catenin junctions were observed in the aberrant epithelial cell population that had migrated apically and now resided near the top of the fiber cells (open arrow). Also, no β-catenin staining could be detected in the nucleus as would occur upon activation of the Wnt signaling pathway. Suppression of Src kinase activation resulted in the maintenance of normal β-catenin cell-cell junctions along lateral borders of the lens epithelium (closed arrow). (C) Western blot analysis of lens epithelium isolated from lenses grown in the presence (P-PP1) and absence (D-DMSO) of the Src kinase inhibitor PP1 at days 1 (D1), 5 (D5), and 10 (D10) of cataract culture using an antibody to the cytoplasmic domain of N-cadherin. E10 is lens epithelium isolated directly from lenses at embryonic day 10. Arrow denotes the 42 kDa N-cadherin cleavage product, which is quantified in (D). There was substantial loss of cadherin junctions from cell-cell borders and increased cleavage of N-cadherin cytoplasmic domain in lenses grown in cataract culture, prevented by suppressing the activation of Src kinases.