![]() Figure 3 of
Ponce, Mol Vis 2006;
12:879-884.
Figure 3 of
Ponce, Mol Vis 2006;
12:879-884.
Figure 3.
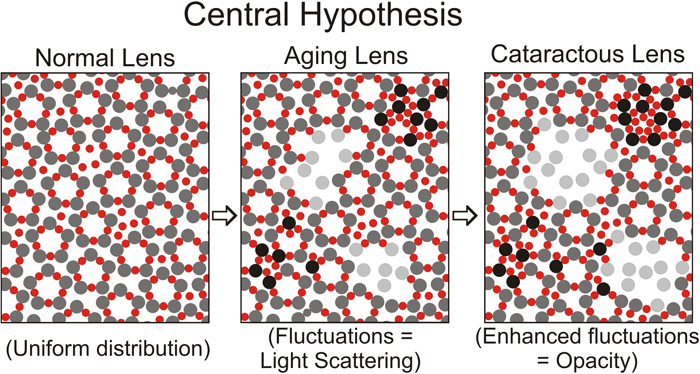
Hypothetical view of protein-protein interactions in the normal young lens, normal aging lens, and cataractous lens. The large spheres (gray) and small spheres (red) represent two different crystallins such as α-crystallin oligomers, β-crystallin oligomers, or γ-crystallin oligomers. To simplify the figure, we have not included a third class of crystallins, which may stabilize short-range interactions as discussed in "Molecular nature of short-range interactions", above. In the normal young lens (left panel), there is uniform spatial distribution of these proteins. In the normal aging lens (center panel), there are fluctuations in this distribution greater than approximately 10% of the wavelength of visible light, due to either a decrease (light gray spheres) or increase (black spheres) in the attractive interactions of the two crystallins. The fluctuations in protein distribution result in fluctuations in refractive index and subsequent scattering of light. In the cataractous lens (right panel), changes in protein distribution and refractive index become more pronounced than in aging, resulting in lens opacification. Also, in the normal aging and cataractous lens, an increase in protein-protein interaction may lead to the formation of very high molecular weight aggregates of at least 50x106 [3], which may directly scatter light.