![]() Figure 1 of
Bando, Mol Vis 2006;
12:692-697.
Figure 1 of
Bando, Mol Vis 2006;
12:692-697.
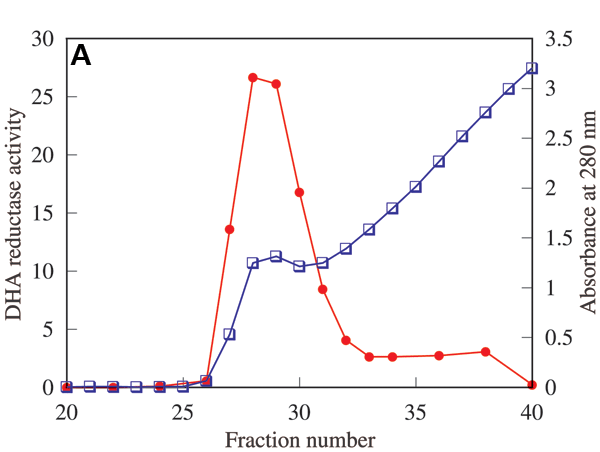
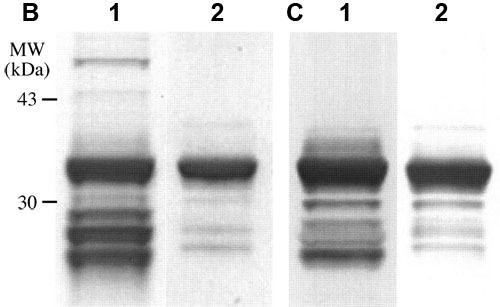
Figure 1. Partial purification of λ-crystallin
A: λ-Crystallin was partially purified by Affi-Gel blue affinity column chromatography of λ/βL1-crystallin fraction separated from the rabbit lens soluble fraction by gel filtration. The λ/βL1-crystallin fraction (14 mg protein) was subjected to a column (1.0 cm in diameter X 7.5 cm in length) of Affi-Gel blue gel. A linear gradient of NADH (0-1 mM) plus NaCl (0-1 M) was started at fraction 26. Fractions were collected in 2 ml aliquots, and measured for absorbances at 280 nm (blue box) and DHA reductase activities (filled red circle). A main fraction of λ-crystallin was found at about 0.25 mM NADH, 0.25 M NaCl. B: SDS-PAGE gels stained with Coomassie brilliant blue, and C: Western blots probed with antiserum to recombinant λ-crystallin, showing results from the affinity column chromatography. Lane 1: λ/βL1-crystallin fraction (2.9 μg protein). Lane 2: partially purified λ-crystallin (0.9 μg protein).