![]() Figure 3 of
Tetreault, Mol Vis 2006;
12:1699-1705.
Figure 3 of
Tetreault, Mol Vis 2006;
12:1699-1705.
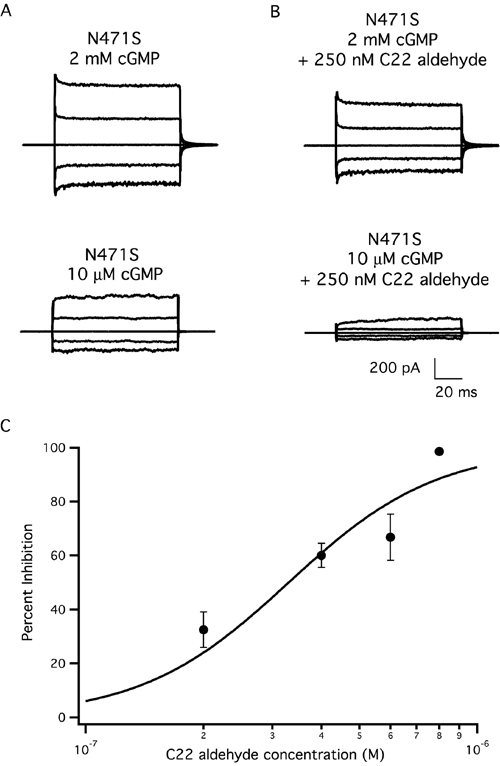
Figure 3. The mutant N471S channel is inhibited by all-trans C22 aldehyde, a retinoid that does not activate rhodopsin
Raw traces representing families of cGMP-activated currents in response to voltage steps ranging from -100 mV to +100 mV in 50 mV increments from a holding potential of 0 mV. Currents measured in the absence of 3',5'-cyclic guanosine monophosphate (cGMP) were subtracted from all traces. A: N471S at 2 mM and 10 μM cGMP. B: Steady state levels of current activation after addition of 250 nM C22 aldehyde on the same patch as in (A). Current at 2 mM is reduced by 29%, whereas the current in 10 μM cGMP has decreased by 56%. C: Percent inhibition of the N471S channels in saturating cGMP (2 mM) by C22 aldehyde. IC50=330 nM, n=2.3. Error bars represent standard error of the mean. (Two to six patches per concentration of C22 aldehyde).