![]() Figure 1 of
Tetreault, Mol Vis 2006;
12:1699-1705.
Figure 1 of
Tetreault, Mol Vis 2006;
12:1699-1705.
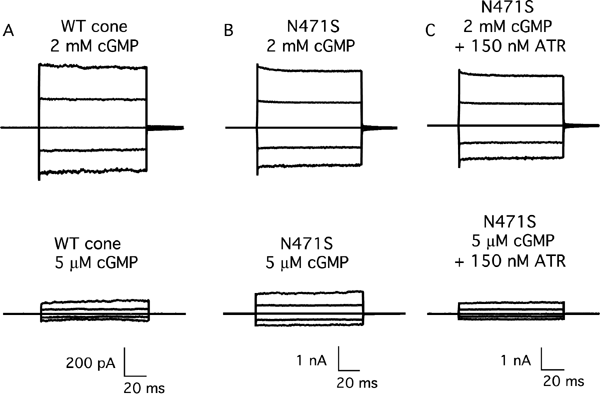
Figure 1. Activation of the mutant N471S channel at physiological cGMP levels can be tuned down toward normal activation by addition of all-trans retinal
Raw traces representing families of cGMP-activated currents from an inside-out patch in response to voltage steps ranging from -100 mV to +100 mV in 50 mV increments from a holding potential of 0 mV. Currents measured in the absence of cGMP were subtracted from all traces. A: Wild-type human CNGA3 channel in 2 mM and 5 μM cGMP. B: Increased activation of the N471S cone channel at 5 μM cGMP. C: Currents decreased at both high and low cGMP upon addition of 150 nM ATR. However, at near physiological cGMP (5 μM), the percent of channel activation became similar to that of the wild-type cone channel. WT cone represents wild-type homomeric cone CNG channel (formed as a tetramer of the human CNGA3 channel subunit); N471S represents a naturally occurring human mutation of CNGA3 associated with cone dystrophy; ATR represents all-trans retinal.