![]() Figure 5 of
Ascano, Mol Vis 2006;
12:1516-1525.
Figure 5 of
Ascano, Mol Vis 2006;
12:1516-1525.
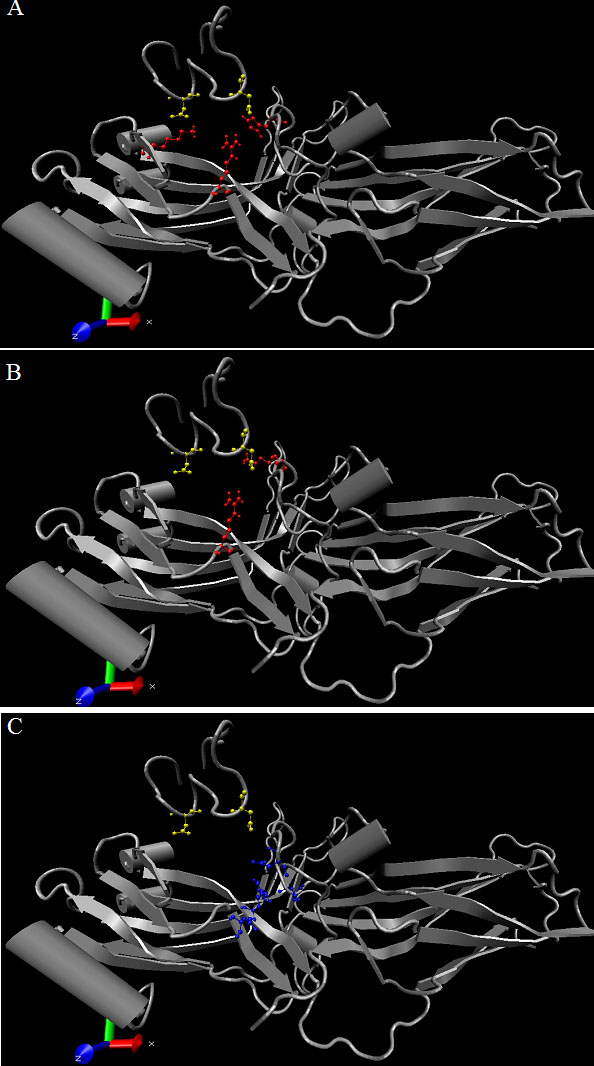
Figure 5. Model for the proposed interactions between arrestin and the phosphorylated rhodopsin cytoplasmic tail
Aspects of this model have been put forth previously by Gurevich and coworkers [44]. Panels are generated using VMD. In this model, arrestin exists alone in a stable low-affinity rhodopsin-binding state due to certain intramolecular interactions that both anchor the arrestin carboxyl tail to the main arrestin body as well as maintain rhodopsin high-affinity binding sites hidden. The role of the phosphorylated residues on the rhodopsin tail is two-fold: (1) displace the arrestin carboxyl tail and (2) generate a conformational change that exposes the high-affinity rhodopsin binding sites. The arrestin carboxyl tail is anchored to the main arrestin body by intramolecular interactions involving His301 and Arg29 (A). Upon displacement of the arrestin carboxyl tail, the negative charges on the rhodopsin tail are initially anchored to arrestin through specific interactions with residues Lys15, Lys300, and Arg29 (B). However, for efficient rhodopsin inhibition the high-affinity rhodopsin binding sites are required, and these are made available upon disruption of the salt-bridges (C) within the polar core, inducing a conformational change in arrestin, revealing the high-affinity binding sites.