![]() Figure 1 of
Ascano, Mol Vis 2006;
12:1516-1525.
Figure 1 of
Ascano, Mol Vis 2006;
12:1516-1525.
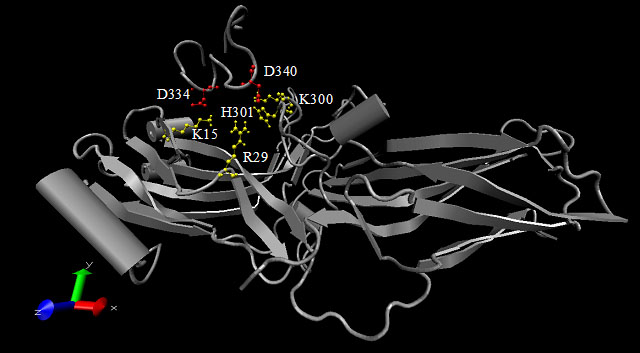
Figure 1. Interactions between arrestin and rhodopsin's cytoplasmic tail
This figure is a representation of the computational simulations of the interactions between arrestin and the rhodopsin cytoplasmic tail. It is a Visual Molecular Dynamics representation [43] of the Monte-Carlo Simulated Annealing simulations previously described in Ling et al. [13]. The interaction between a 20 amino acid rhodopsin C-terminal peptide analog and the arrestin molecule was computationally simulated. The phosphorylatable sites on the peptide were replaced with Asp residues to mimic phospho-residues. This figure depicts the final conformation of both the peptide and arrestin once the simulations were complete. The residues on the rhodopsin peptide designated in red are residues Asp334 and Asp340 that were found to have a positive interaction with the residues on arrestin, designated in yellow. The yellow residues on arrestin are residues Lys15, Arg29, His301, and Lys300.