![]() Figure 6 of
Singh, Mol Vis 2006;
12:1372-1379.
Figure 6 of
Singh, Mol Vis 2006;
12:1372-1379.
Figure 6.
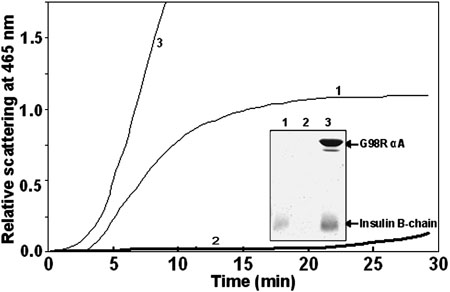
G98R mutation in αA-crystallin leads to loss of chaperone-like activity towards DTT-induced aggregation of insulin. Aggregation of insulin (0.2 mg/ml), as monitored by light scattering at 465 nm, was initiated by the addition of 20 mM DTT in the absence (curve 1) or in the presence of 0.2 mg/ml wild type (curve 2) or the mutant (curve 3) αA-crystallin. Inset shows that the mutant protein co-aggregates with the insulin B-chain as analyzed by SDS-PAGE. The pellet obtained after centrifuging (at 10000x g for 20 min) the samples (after DTT-induced aggregation for 30 min) was subjected to electrophoresis on a 17% SDS-Polyacrylamide gel: Samples are insulin alone (lane 1), insulin in the presence of the wild type (lane 2), or the mutant (lane 3) αA-crystallin.