![]() Figure 3 of
Acosta, Mol Vis 2005;
11:717-728.
Figure 3 of
Acosta, Mol Vis 2005;
11:717-728.
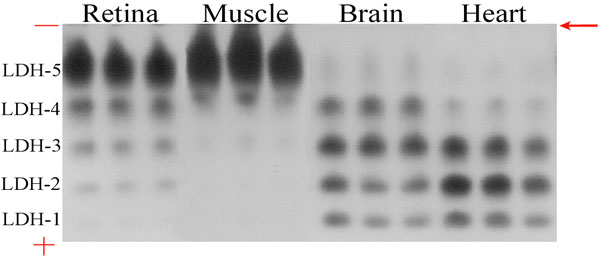
Figure 3. Native gel electrophoresis of LDH isoenzymes in control mouse tissues
The LDH isoenzyme ratio of the brain tissue was similar to ratios found in the aerobic heart tissue that expresses predominantly isoenzymes LDH1-LDH3 and low amounts of the LDH5 isoform. Adult tissues from the anaerobic skeletal muscle express largely the LDH5 isoenzyme. The retina has a similar prevalence of the skeletal muscle-type isoforms in addition to very low amounts of LDH1. The tissue samples were diluted so that equal amounts of LDH activity were loaded into the gel. A volume of 20 μl of each diluted supernatant was added into each electrophoresis gel well. The gel was run at a constant 100 volts from negative to positive for 1.5-2 h or until the bromophenol blue had run out of the gel. Upon the run, the highly colored nitrobluetetrazolium-formazan product localized on zones of LDH activity and the amount of blue color formed was quantitatively related to the level of LDH isoenzyme present. The mobility of the isoenzymes were governed by their individual size and charge. LDH-5, the skeletal muscle isoenzyme migrates the least from the starting point (indicated with an arrow) whereas LDH-1, the heart isoenzyme moves the farthest towards the anode (+) as it has the most positive charge. LDH-2, LDH-3 and LDH-4 migrate towards the anode in order of decreasing anodic mobilities.