![]() Figure 4 of
Karan, Mol Vis 2005;
11:657-664.
Figure 4 of
Karan, Mol Vis 2005;
11:657-664.
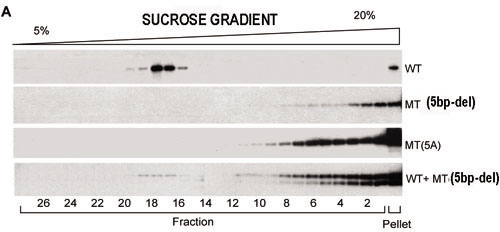
Figure 4. Sucrose density gradient sedimentation and densitometric analysis of ELOVL4 aggregates
A: Western blots show immunolabeled wtEGFP/ELOVL4 and mutant ELOVL4 protein aggregates fractionated by sucrose density gradients. Cells were transfected with wtEGFP/ELOVL4, mtEGFP/ELOVL4(5bp-del), or mtEGFP/ELOVL4(5A) or co-transfected with wtEGFP/ELOVL4 and mtEGFP/ELOVL4(5bp-del). wtEGFP/ELOVL4 signal was normally found in lower molecular weight fractions. The 5bp-del mutant and 5A mutant shifted to the lower fraction numbers indicating formation of larger molecular weight aggregates. Mutant ELOVL4 (mtEGFP/ELOVL4(5bp-del)) induced the wild-type protein (wtEGFP/ELOVL4) to shift to the higher molecular weight fractions. Co-transfected cell lysates showed strong immunolabeling in higher molecular weight fractions similar to that of single-mutant transfections. B: Fractionated ELOVL4 proteins (wtEGFP/ELOVL4, 5bp-del mutant, and 5A mutant) and co-transfected wtEGFP/ELOVL4 and mtEGFP/ELOVL4(5bp-del), measured by densitometry of western blots, are graphically represented.