![]() Figure 4 of
Houdart, Mol Vis 2005;
11:1061-1070.
Figure 4 of
Houdart, Mol Vis 2005;
11:1061-1070.
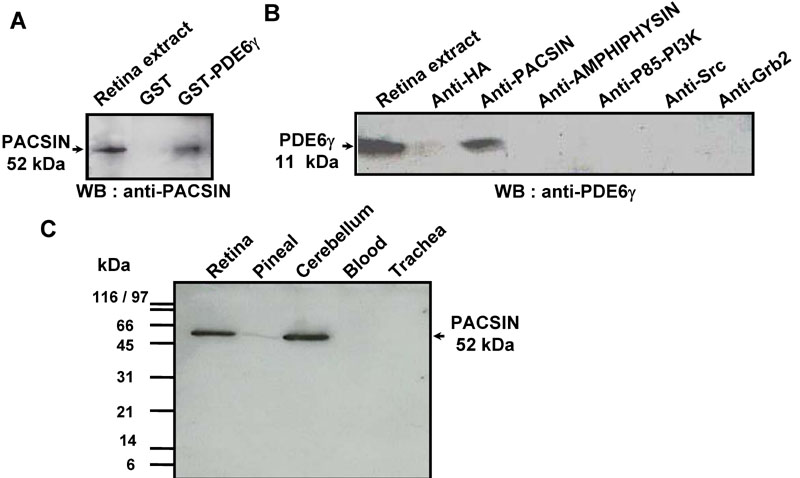
Figure 4. PDE6γ-pacsin in vivo interaction
A: Retinas obtained in the middle of the day were homogenized in Bramph buffer and an aliquot containing 400 μg of protein was incubated overnight with glutathione-sepharose beads sensitized with either GST or the GST-PDE6γ fusion protein. After washes, the material bound was eluted in 50 μl of Laemmli buffer and a 10 μl aliquot was analyzed on a western blot with anti-PACSIN antibody, using the ECL method. Comparison with the "input" lane corresponding to 50 μg of retinal proteins indicates PACSIN was pulled-down with about 12.5% efficiency. Probing the western blot with antibodies to AMPHIPHYSIN, P85-PI3K, Grb2 or Src gave negative results (not illustrated). B: An aliquot of retinal homogenates (400 μg of protein) was incubated overnight with either anti-PACSIN antibody (5 μg) or anti-HA antibody (5 μg, control) and a slurry of protein A sepharose. After washes, the bound material was eluted in Laemmli buffer and analyzed on western blot with anti-PDE6γ antibody, using the ECL method. Similar immunoprecipitation procedures with antibodies to AMPHIPHYSIN, P85-PI3K, Src or Grb2 failed to co-precipitate PDE6γ. C: PACSIN expression was analyzed in different tissues: retina, pineal, cerebellum, blood, and trachea. Proteins (20 μg) of each tissue were analyzed by SDS-PAGE and western blotting as described in material and methods.