![]() Figure 1 of
White, Mol Vis 2004;
10:738-749.
Figure 1 of
White, Mol Vis 2004;
10:738-749.
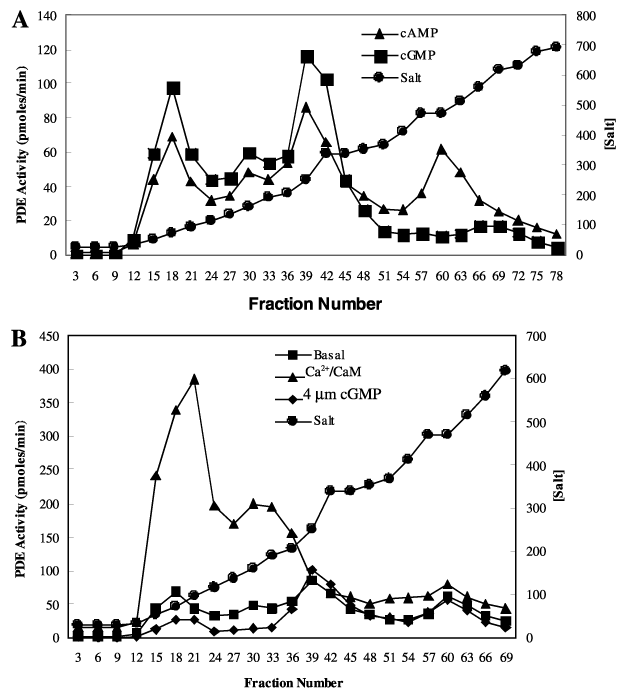
Figure 1. Chromatographic fractionation of PDE activity in Y79 Rb cells
A: Hydrolysis of cGMP and cAMP. PDE activity in total soluble Y79 protein extracts was fractionated in DEAE-Trisacryl M and eluted with a 0-0.8 M NaOAC salt gradient. The salt concentration is given in mM, and based on conductivity measurements of each fraction compared to a standard curve. PDE activity was measured every third fraction with 0.5 μM 3H-cGMP or 0.25 μM 3H-cAMP as described in "Materials and Methods". Three peaks of activity were identified and pooled for kinetic, drug, and Ca2+/calmodulin (CaM) activation studies (peak 1, fractions 14-22; peak 2, 29-46; peak 3, 55-66). The PDE in peaks 1 and 2 showed almost no preference for cAMP or cGMP as substrate. Peak 3 showed a marked preference for cAMP as substrate. B: Ca2+/Calmodulin stimulation of PDE activity. Each fraction was assayed for activity in the presence and absence of 0.5 μM cGMP or 2 mM CaCl2 and 100 ng CaM with 0.25 μM 3H-cAMP. Peaks 1 and 2 activities were stimulated 5-10 fold by Ca2+/CaM and were slightly inhibited by cGMP and peak 3 activity was unaffected by the presence of cGMP or Ca2+/CaM.