 A Molecular Vision Short Report
A Molecular Vision Short Report
Peptide Sequences for ß-Crystallins of a Teleost Fish
Graeme Wistow
Section on Molecular Structure and Function
LMDB, National Eye Institute,
National Institutes of Health, Bethesda, MD 20892 USA
graeme@mge2.nei.nih.gov
The gradients of refractive index in vertebrate lenses are achieved through varying expression of multiple crystallins during development (2, 5). Much of this variability is due to the ß-crystallins. In both mammals and birds, six ß-crystallin genes give rise to seven highly conserved polypeptides (1, 3, 4). These proteins fall into two subfamilies, ßA (ßA3/A1, ßA2, ßA4) and ßB (ßB1, ßB2, ßB3), which have characteristic sequence features. Recently there has been some discussion of the evolutionary origins of these subfamilies (3, 4). The conservation of these ß-crystallins in mammals and birds shows that they were already established at least 300 Myr ago. As discussed by de Jong et al. (3), the presence of amphibian homologues of ßA3/A1 in Rana (6) and ßA4 in Xenopus (Lubsen, unpublished) dates the ßA subfamily to at least 350 Myr. However no sequence data for ßB-crystallins have been reported from amphibians or for ß-crystallins from fish. As a contribution to this discussion this note describes sequences of several crystallin peptides obtained from a surf perch (Embiotocidae sp.), a marine teleost fish, some of which are characteristic of the ßB family and suggest that this branch of the family is at least 400 Myr old.
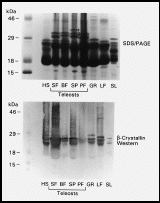 A survey of lenses from fish of four groups, (Elasmobranchii [shark], Teleostei [batfish, surf perch, sun fish, puffer fish], Holostei [gar], Dipnoi [lungfish]), and Necturus maculosus [salamander] by SDS PAGE (7) revealed prominent subunits with apparent sizes greater than 30 kDa in soluble lens extracts of teleost fish (Fig. 1, top panel). This size range is typical of many taxon-specific crystallins but larger than expected for alpha, ß and gamma-crystallin subunits (2, 5). Furthermore, although western blot analysis with rabbit anti-mouse ß-crystallin antiserum (8) detected ß-crystallins in the usual 22-30 kDa size range in all species examined, the larger sized bands in teleosts did not react with the antiserum (Fig. 1, bottom panel). Since this raised the interesting possibility that these bands represented novel taxon-specific crystallins they were investigated by microsequencing.
A survey of lenses from fish of four groups, (Elasmobranchii [shark], Teleostei [batfish, surf perch, sun fish, puffer fish], Holostei [gar], Dipnoi [lungfish]), and Necturus maculosus [salamander] by SDS PAGE (7) revealed prominent subunits with apparent sizes greater than 30 kDa in soluble lens extracts of teleost fish (Fig. 1, top panel). This size range is typical of many taxon-specific crystallins but larger than expected for alpha, ß and gamma-crystallin subunits (2, 5). Furthermore, although western blot analysis with rabbit anti-mouse ß-crystallin antiserum (8) detected ß-crystallins in the usual 22-30 kDa size range in all species examined, the larger sized bands in teleosts did not react with the antiserum (Fig. 1, bottom panel). Since this raised the interesting possibility that these bands represented novel taxon-specific crystallins they were investigated by microsequencing.
Three fractions of proteins from surf perch lens, designated SP1,2,3 in descending size order, were isolated by SDS PAGE, western blotting and excision of Ponceau S stained bands, as described before (7). These bands were digested by trypsin and peptides were separated by HPLC and subjected to automated sequencing by the Harvard Microchemistry Facility (7). Surprisingly all the peptides sequenced showed close similarity to ß-crystallins (Fig. 2). Their unusual mobility and lack of immunoreactivity may be due to unknown post-translational modifications which seem to be widespread in teleosts but absent from some other aquatic vertebrates.
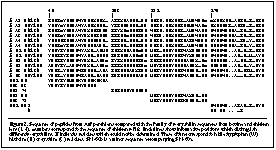
The closest matches were for two peptides from different size fractions, SP2-95 and SP3-72, which were both almost identical to bovine and chicken ßB3-crystallin sequences. Interestingly, both SP2-95 and SP3-72 closely match the same part of ßB3. Since they were derived from the same lens this suggests the presence of more than one ßB3-like subunit in these lenses, or possibly divergent alleles in one individual. Where SP2-95 differs from tetrapod ßB3 sequences it contains a tyrosine (Y) more typical of ßB1 (Fig. 2). Similarly the minor sequence SP1-60b had features characteristic of ßB2-crystallins (Fig. 2). However other peptides were less clearly related to specific bovine and chicken sequences. For example, SP1-60a was about equally similar to ßB1 and ßB3 while SP1-95 was about equally similar to ßB3 and ßA2 (Table).
TABLE:
Identities between surf perch peptides and bovine and chicken ß-crystallins. Total number of positions counted are shown below each column. Gaps are counted as informative positions.
| Crystallin |
SP1-95 |
SP3-68 |
SP2-74 |
SP2-95 |
SP3-72 |
SP1-60a |
SP1-60b |
| ßA2 Chick |
10 |
10 |
4 |
8 |
7 |
6 |
1 |
| ßA2 Bovine |
11 |
9 |
5 |
7 |
7 |
6 |
2 |
| ßA3 Chick |
8 |
7 |
4 |
9 |
8 |
10 |
2 |
| ßA3 Bovine |
9 |
8 |
5 |
9 |
8 |
10 |
2 |
| ßA4 Chick |
9 |
9 |
7 |
8 |
7 |
8 |
2 |
| ßA4 Bovine |
9 |
9 |
6 |
8 |
7 |
8 |
2 |
| ßB1 Chick |
9 |
10 |
4 |
15 |
12 |
13 |
4 |
| ßB1 Bovine |
10 |
10 |
6 |
15 |
13 |
13 |
4 |
| ßB2 Chick |
9 |
10 |
5 |
11 |
11 |
11 |
7 |
| ßB2 Bovine |
9 |
9 |
5 |
11 |
11 |
11 |
6 |
| ßB3 Chick |
12 |
12 |
8 |
17 |
17 |
12 |
4 |
| ßB3 Bovine |
10 |
10 |
7 |
18 |
17 |
13 |
3 |
|
| Total Position |
17 |
16 |
12 |
19 |
18 |
18 |
8 |
These results show that some proteins characteristic of the ßB-crystallin subfamily are expressed in the lenses of teleost fish whose lineage diverged from that of tetrapods more than 400 Myr ago. However, it is not clear whether direct homologues of the six ß-crystallin genes of mammals and birds exist in fish. The limited data reported here suggest the possibility of independent histories of ß-crystallin gene duplication following the divergence of teleost fish and tetrapods since, as can be seen in Fig. 2, several fish lens peptides contain residues which in mammals and birds are specific to different ß-crystallins. It appears that the gene duplications which gave rise to the modern subfamilies of ß-crystallins began at a very early stage in the evolution of the vertebrate lens. This may have conferred a powerful mechanism for modifying the optical properties of the developing lens which has been conserved ever since.
Acknowledgements
I am grateful to Hyong S. Kim for providing fish and salamander lenses.
References
1. Berbers GA, Hoekman WA, Bloemendal H, de Jong WW, Kleinschmidt T, Braunitzer G. Homology between the primary structures of the major bovine ßcrystallin chains. Eur J Biochem;139 (1984) 467-479.
2. de Jong WW. Evolution of lens and crystallins in Bloemendal H (ed) , Molecular and Cellular Biology of the Eye Lens, vol . WileyInterscience. New York (1981) pp. 221-278.
3. de Jong WW, Lubsen NH, Kraft HJ. Molecular Evolution of the Eye Lens in Chader G, Osbourne N (eds) , Progress in Retinal and Eye Research, vol 13. Elsevier Science Ltd. (1994) pp. 391-442.
4. Duncan MK, BanerjeeBasu S, McDermott JB, Piatigorsky J. Sequence and expression of chicken ßA2 and ßB3crystallin. Exp Eye Res (1995) submitted.
5. Harding JJ, Crabbe MJC. The lens: Development, proteins, metabolism and cataract in Davson H (ed) , The Eye, vol 1B. Academic Press. New York (1984) pp. 207-492.
6. Luchin SV, Zinovieva RD, Tomarev SI, Dolgilevich SM, Gause GG Jr, Bax B, Driessen H, Blundell TL. Frog lens ßA1crystallin: the nucleotide sequence of the cloned cDNA and computer graphics modelling of the threedimensional structure. Biochim Biophys Acta;916 (1987) 163-171.
7. Wistow G. Lens crystallins: a model system for gene recruitment in Zimmer EA, White TJ, Cann RL, Wilson AC (eds) Methods in Enzymology, Molecular Evolution. Producing the Biochemical Data, vol 224. Academic Press. San Diego, CA (1993) pp. 563-575.
8. Zigler JS, Carper DA, Kinoshita JH. Changes in lens crystallins during cataract development in the Philly mouse. Ophthal Res;13 (1981) 237-251.
 Return to Molecular Vision homepage
Return to Molecular Vision homepage
Received 21 July 1995 | Accepted 5 October 1995 | Uploaded 11 October 1995
Referencing Note: This article may be referenced as: Mol. Vis. 1:1, 1995.
Alternatively, this article may be referenced by its unique URL: http://www.emory.edu/molvis/v1/wistow
© 1995 Molecular Vision
 A Molecular Vision Short Report
A Molecular Vision Short Report
 A survey of lenses from fish of four groups, (Elasmobranchii [shark], Teleostei [batfish, surf perch, sun fish, puffer fish], Holostei [gar], Dipnoi [lungfish]), and Necturus maculosus [salamander] by SDS PAGE (7) revealed prominent subunits with apparent sizes greater than 30 kDa in soluble lens extracts of teleost fish (Fig. 1, top panel). This size range is typical of many taxon-specific crystallins but larger than expected for alpha, ß and gamma-crystallin subunits (2, 5). Furthermore, although western blot analysis with rabbit anti-mouse ß-crystallin antiserum (8) detected ß-crystallins in the usual 22-30 kDa size range in all species examined, the larger sized bands in teleosts did not react with the antiserum (Fig. 1, bottom panel). Since this raised the interesting possibility that these bands represented novel taxon-specific crystallins they were investigated by microsequencing.
A survey of lenses from fish of four groups, (Elasmobranchii [shark], Teleostei [batfish, surf perch, sun fish, puffer fish], Holostei [gar], Dipnoi [lungfish]), and Necturus maculosus [salamander] by SDS PAGE (7) revealed prominent subunits with apparent sizes greater than 30 kDa in soluble lens extracts of teleost fish (Fig. 1, top panel). This size range is typical of many taxon-specific crystallins but larger than expected for alpha, ß and gamma-crystallin subunits (2, 5). Furthermore, although western blot analysis with rabbit anti-mouse ß-crystallin antiserum (8) detected ß-crystallins in the usual 22-30 kDa size range in all species examined, the larger sized bands in teleosts did not react with the antiserum (Fig. 1, bottom panel). Since this raised the interesting possibility that these bands represented novel taxon-specific crystallins they were investigated by microsequencing.

 Return to Molecular Vision homepage
Return to Molecular Vision homepage